Classical Heisenberg model: Difference between revisions
Jump to navigation
Jump to search
en>Headbomb Unlinked: Heisenberg model |
en>Anrnusna No edit summary |
||
| Line 1: | Line 1: | ||
This page provides supplementary chemical data on [[toluene]]. | |||
==MSDS sheets== | |||
* [http://hazard.com/msds/mf/baker/baker/files/t3913.htm Baker] | |||
==Structure and properties== | |||
{| border="1" cellspacing="0" cellpadding="3" style="margin: 0 0 0 0.5em; background: #FFFFFF; border-collapse: collapse; border-color: #C0C090;" | |||
! {{chembox header}} | Structure and properties | |||
|- | |||
| [[Index of refraction]], ''n''<sub>D</sub> | |||
| 1.4969 at 20°C <!-- Please omit if not applicable --> | |||
|- | |||
| [[Abbe number]] | |||
|? <!-- Please omit if not applicable --> | |||
|- | |||
| [[Dielectric constant]], ε<sub>r</sub> | |||
| 2.379 ε<sub>0</sub> at 25°C | |||
|- | |||
| [[Bond strength]] | |||
| ? <!-- Specify which bond. Please omit if not applicable --> | |||
|- | |||
| [[Bond length]] | |||
| ? <!-- Specify which bond. Please omit if not applicable --> | |||
|- | |||
| [[Bond angle]] | |||
| ? <!-- Specify which angle, e.g. Cl-P-O. Please omit if not applicable --> | |||
|- | |||
| [[Magnetic susceptibility]] | |||
| ? <!-- Please omit if not applicable --> | |||
|- | |||
| [[Surface tension]] | |||
| 28.52 dyn/cm at 25°C | |||
|- | |||
| [[Viscosity]]<ref name="cheric_p">{{Cite web|url=http://www.cheric.org/research/kdb/hcprop/cmpsrch.php|title=Pure Component Database|publisher=Chemical Engineering Research Information Center|format=Queriable database|accessdate=12 May 2007}}</ref> | |||
| | |||
{| | |||
|- | |||
| 1.1813 mPa·s || at –25°C | |||
|- | |||
| 1.0787 mPa·s || at –20°C | |||
|- | |||
| 0.9888 mPa·s || at –15°C | |||
|- | |||
| 0.9095 mPa·s || at –10°C | |||
|- | |||
| 0.8393 mPa·s || at –5°C | |||
|- | |||
| 0.7770 mPa·s || at 0°C | |||
|- | |||
| 0.7214 mPa·s || at 5°C | |||
|- | |||
| 0.6717 mPa·s || at 10°C | |||
|- | |||
| 0.6270 mPa·s || at 15°C | |||
|- | |||
| 0.5867 mPa·s || at 20°C | |||
|- | |||
| 0.5503 mPa·s || at 25°C | |||
|- | |||
| 0.5173 mPa·s || at 30°C | |||
|- | |||
| 0.4873 mPa·s || at 35°C | |||
|- | |||
| 0.4599 mPa·s || at 40°C | |||
|- | |||
| 0.4349 mPa·s || at 45°C | |||
|- | |||
| 0.4120 mPa·s || at 50°C | |||
|- | |||
|} | |||
|- | |||
|} | |||
== Thermodynamic properties == <!-- KEEP this header, it is linked to from the infobox on the main article page --> | |||
{| border="1" cellspacing="0" cellpadding="6" style="margin: 0 0 0 0.5em; background: #FFFFFF; border-collapse: collapse; border-color: #C0C090;" | |||
! {{chembox header}} | Phase behavior | |||
|- | |||
| [[Triple point]] | |||
| 178.15 K (−94.99 °C), ? Pa | |||
|- | |||
| [[Critical point (thermodynamics)|Critical point]] | |||
| 591.79 K (318.64 °C), 4.109 MPa | |||
|- | |||
| [[Standard enthalpy change of fusion|Std enthalpy change<br/>of fusion]]Δ<sub>fus</sub>''H''<sup><s>o</s></sup> | |||
| 6.636 kJ/mol | |||
|- | |||
| [[Standard entropy change of fusion|Std entropy change<br/>of fusion]]Δ<sub>fus</sub>''S''<sup><s>o</s></sup> | |||
| 37.25 J/(mol·K) | |||
|- | |||
| [[Standard enthalpy change of vaporization|Std enthalpy change<br/>of vaporization]]Δ<sub>vap</sub>''H''<sup><s>o</s></sup> | |||
| 38.06 kJ/mol | |||
|- | |||
| [[Standard entropy change of vaporization|Std entropy change<br/>of vaporization]]Δ<sub>vap</sub>''S''<sup><s>o</s></sup> | |||
| 87.30 J/(mol·K) | |||
|- | |||
! {{chembox header}} | Solid properties | |||
|- | |||
| [[Standard enthalpy change of formation|Std enthalpy change<br/>of formation]] Δ<sub>f</sub>''H''<sup><s>o</s></sup><sub>solid</sub> | |||
| ? kJ/mol | |||
|- | |||
| [[Standard molar entropy]]<br/>''S''<sup><s>o</s></sup><sub>solid</sub> | |||
| ? J/(mol K) | |||
|- | |||
| [[Heat capacity]] ''c<sub>p</sub> <sub>liquid</sub>'' | |||
| 181,2 J/(mol K) | |||
|- | |||
| [[Heat capacity]] ''c<sub>p</sub> <sub>gas</sub>'' | |||
| 103,6 J/(mol K) | |||
|- | |||
! {{chembox header}} | Liquid properties | |||
|- | |||
| [[Standard enthalpy change of formation|Std enthalpy change<br/>of formation]] Δ<sub>f</sub>''H''<sup><s>o</s></sup><sub>liquid</sub> | |||
| +12.0 kJ/mol | |||
|- | |||
| [[Standard molar entropy]]<br/>''S''<sup><s>o</s></sup><sub>liquid</sub> | |||
| 220.96 J/(mol K) | |||
|- | |||
| [[Heat capacity]] ''c<sub>p</sub>'' | |||
| 155.96 J/(mol K) | |||
|- | |||
! {{chembox header}} | Gas properties | |||
|- | |||
| [[Standard enthalpy change of formation|Std enthalpy change<br/>of formation]] Δ<sub>f</sub>''H''<sup><s>o</s></sup><sub>gas</sub> | |||
| +50.00 kJ/mol | |||
|- | |||
| [[Standard molar entropy]]<br/>''S''<sup><s>o</s></sup><sub>gas</sub> | |||
| ? J/(mol K) | |||
|- | |||
| [[Heat capacity]] ''c<sub>p</sub>'' | |||
| 103.7 J/(mol K) | |||
|- | |||
| [[van der Waals equation|van der Waals' constants]]<ref name="lange1522">''Lange's Handbook of Chemistry'' 10th ed, pp 1522-1524</ref> | |||
| a = 2438 L<sup>2</sup> kPa/mol<sup>2</sup><br> b = 0.1463 liter per mole | |||
|- | |||
|} | |||
==Vapor pressure of liquid== | |||
{| border="1" cellspacing="0" cellpadding="6" style="margin: 0 0 0 0.5em; background: white; border-collapse: collapse; border-color: #C0C090;" | |||
|- | |||
| {{chembox header}} | '''P in mm Hg''' || 1 || 10 || 40 || 100 || 400 || 760 || 1520 || 3800 || 7600 || 15200 || 30400 || 45600 | |||
|- | |||
| {{chembox header}} | '''T in °C''' || -25 || 6.4 || 31.8 || 51.9 || 89.5 || 110.6 || 136.5 || 178.0 || 215.8 || 262.5 || 319.0 || — | |||
|} | |||
Table data obtained from ''CRC Handbook of Chemistry and Physics'' 44th ed. | |||
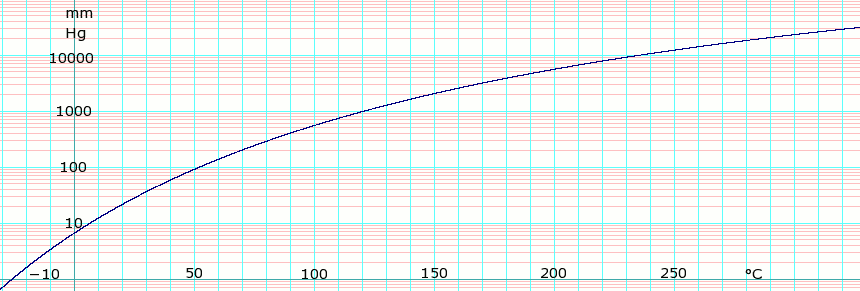
[[Image:logTolueneVaporPressure.png|thumb|860px|left|'''log<sub>10</sub> of Toluene vapor pressure.''' Uses formula: <math>\scriptstyle \log_{10} P_{mmHg} = 6.95464 - \frac {1344.8} {T+219.482}</math> obtained from ''Lange's Handbook of Chemistry'', 10th ed.]]{{Clear}} | |||
==Spectral data== | |||
{| border="1" cellspacing="0" cellpadding="3" style="margin: 0 0 0 0.5em; background: #FFFFFF; border-color: #C0C090;" | |||
! width="85%" align="center" cellspacing="3" style="font-size:95%; border: 1px solid #C0C090; background-color: #F8EABA; margin-bottom: 3px;" colspan="2" | [[UV/VIS spectroscopy|UV-Vis]] | |||
|- | |||
| Spectrum | |||
| [http://webbook.nist.gov/cgi/cbook.cgi?ID=C108883&Units=SI&Mask=400#UV-Vis-Spec NIST] | |||
|- | |||
| [[Lambda-max]] | |||
| 253, 259, 261, 268[[Nanometre|nm]] | |||
|- | |||
| Log [[molar absorptivity|Ε]] | |||
| 2.36, 2.42, 2.43, 2.27 | |||
|- | |||
! {{Chembox header}} colspan="2" | [[Infrared|IR]] | |||
|- | |||
| Spectrum | |||
| [http://webbook.nist.gov/cgi/cbook.cgi?ID=C108883&Units=SI&Type=IR-SPEC&Index=2#IR-SPEC NIST] | |||
|- | |||
| Major absorption bands | |||
| 3028, 1605, 1496, 729, 696 cm<sup>−1</sup> | |||
|- | |||
! {{Chembox header}} colspan="2" | [[NMR Spectroscopy|NMR]] | |||
|- | |||
| [[Proton NMR]] | |||
| (CDCl<sub>3</sub>, 300 MHz) δ 7.17-7.11 (m, 2H), 7.08-7.01 (m, 3H), 2.32 (s, 3H) | |||
|- | |||
| [[Carbon-13 NMR]] <!-- Link to image of spectrum --> | |||
| (CDCl<sub>3</sub>, 100 MHz) δ 137.7, 128.7, 127.9, 125.0, 20.8 | |||
|- | |||
| Other NMR data <!-- Insert special data e.g. <sup>19</sup>F chem. shifts, omit if not used --> | |||
| ? | |||
|- | |||
! width="85%" align="center" cellspacing="3" style="font-size:95%; border: 1px solid #C0C090; background-color: #F8EABA; margin-bottom: 3px;" colspan="2" | [[Mass Spectrometry|MS]] | |||
|- | |||
| Masses of <br>main fragments | |||
| ? <!-- Give list of major fragments --> | |||
|- | |||
| {{Chembox header}} colspan="2" | <small>Except where noted otherwise, data are given for<br>[[standard ambient temperature and pressure|standard ambient temperature and pressure (25°C, 101.3 kPa)]]<br/>[[wikipedia:Chemical infobox|Disclaimer and references]]</small> | |||
|} | |||
==References== | |||
<references/> | |||
[http://webbook.nist.gov/cgi/cbook.cgi?Name=toluene&Units=SI NIST website] | |||
Except where noted otherwise, data relate to [[standard ambient temperature and pressure]]. | |||
[[wikipedia:Chemical infobox|Disclaimer]] applies. | |||
{{Use dmy dates|date=September 2010}} | |||
{{DEFAULTSORT:Toluene (Data Page)}} | |||
[[Category:Chemical data pages]] | |||
Revision as of 20:35, 23 September 2013
This page provides supplementary chemical data on toluene.
MSDS sheets
Structure and properties
| Structure and properties | |||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Index of refraction, nD | 1.4969 at 20°C | ||||||||||||||||||||||||||||||||
| Abbe number | ? | ||||||||||||||||||||||||||||||||
| Dielectric constant, εr | 2.379 ε0 at 25°C | ||||||||||||||||||||||||||||||||
| Bond strength | ? | ||||||||||||||||||||||||||||||||
| Bond length | ? | ||||||||||||||||||||||||||||||||
| Bond angle | ? | ||||||||||||||||||||||||||||||||
| Magnetic susceptibility | ? | ||||||||||||||||||||||||||||||||
| Surface tension | 28.52 dyn/cm at 25°C | ||||||||||||||||||||||||||||||||
| Viscosity[1] |
|
Thermodynamic properties
| Phase behavior | |
|---|---|
| Triple point | 178.15 K (−94.99 °C), ? Pa |
| Critical point | 591.79 K (318.64 °C), 4.109 MPa |
| Std enthalpy change of fusionΔfusH |
6.636 kJ/mol |
| Std entropy change of fusionΔfusS |
37.25 J/(mol·K) |
| Std enthalpy change of vaporizationΔvapH |
38.06 kJ/mol |
| Std entropy change of vaporizationΔvapS |
87.30 J/(mol·K) |
| Solid properties | |
| Std enthalpy change of formation ΔfH |
? kJ/mol |
| Standard molar entropy S |
? J/(mol K) |
| Heat capacity cp liquid | 181,2 J/(mol K) |
| Heat capacity cp gas | 103,6 J/(mol K) |
| Liquid properties | |
| Std enthalpy change of formation ΔfH |
+12.0 kJ/mol |
| Standard molar entropy S |
220.96 J/(mol K) |
| Heat capacity cp | 155.96 J/(mol K) |
| Gas properties | |
| Std enthalpy change of formation ΔfH |
+50.00 kJ/mol |
| Standard molar entropy S |
? J/(mol K) |
| Heat capacity cp | 103.7 J/(mol K) |
| van der Waals' constants[2] | a = 2438 L2 kPa/mol2 b = 0.1463 liter per mole |
Vapor pressure of liquid
| P in mm Hg | 1 | 10 | 40 | 100 | 400 | 760 | 1520 | 3800 | 7600 | 15200 | 30400 | 45600 |
| T in °C | -25 | 6.4 | 31.8 | 51.9 | 89.5 | 110.6 | 136.5 | 178.0 | 215.8 | 262.5 | 319.0 | — |
Table data obtained from CRC Handbook of Chemistry and Physics 44th ed.
50 year old Petroleum Engineer Kull from Dawson Creek, spends time with interests such as house brewing, property developers in singapore condo launch and camping. Discovers the beauty in planing a trip to places around the entire world, recently only coming back from .
Spectral data
| UV-Vis | |
|---|---|
| Spectrum | NIST |
| Lambda-max | 253, 259, 261, 268nm |
| Log Ε | 2.36, 2.42, 2.43, 2.27 |
| IR | |
| Spectrum | NIST |
| Major absorption bands | 3028, 1605, 1496, 729, 696 cm−1 |
| NMR | |
| Proton NMR | (CDCl3, 300 MHz) δ 7.17-7.11 (m, 2H), 7.08-7.01 (m, 3H), 2.32 (s, 3H) |
| Carbon-13 NMR | (CDCl3, 100 MHz) δ 137.7, 128.7, 127.9, 125.0, 20.8 |
| Other NMR data | ? |
| MS | |
| Masses of main fragments |
? |
| Except where noted otherwise, data are given for standard ambient temperature and pressure (25°C, 101.3 kPa) Disclaimer and references | |
References
- ↑ Template:Cite web
- ↑ Lange's Handbook of Chemistry 10th ed, pp 1522-1524
Except where noted otherwise, data relate to standard ambient temperature and pressure.
Disclaimer applies.
30 year-old Entertainer or Range Artist Wesley from Drumheller, really loves vehicle, property developers properties for sale in singapore singapore and horse racing. Finds inspiration by traveling to Works of Antoni Gaudí.
