Spectral set: Difference between revisions
typo: "constructed form functions" -> "constructed from functions" |
en>Thesquaregroot mNo edit summary |
||
| Line 1: | Line 1: | ||
[[Image:Copper K Rontgen.png|thumb | Atomic levels involved in copper Kα and Kβ emission]] | |||
In [[X-ray spectroscopy]], '''K-alpha''' emission lines result when an electron transitions to the innermost "K" shell (principal quantum number 1) from a 2p orbital of the second or "L" shell (with principal quantum number 2). The line is actually a doublet, with slightly different energies depending on [[spin-orbit interaction]] energy between the electron spin and the orbital momentum of the 2p orbital. K-alpha is typically by far the strongest X-ray spectral line for an element bombarded with energy sufficient to cause maximally intense X-ray emission. | |||
The analogous K-alpha spectra line in hydrogen is known as [[Lyman alpha]]; however because of hydrogen's small nuclear charge, this line is in the ultraviolet, not the X-ray range. See [[Siegbahn notation]] for the newer [[IUPAC]]-recommended spectral notation system. | |||
An example of K-alpha lines are those seen for iron as iron atoms radiating X-rays spiralling into a [[black hole]] at the center of a galaxy [http://arxiv.org/abs/astro-ph/0405337]. For such purposes, the energy of the line is adequately calculated to 2-digit accuracy by the use of [[Moseley's law]]: <math>E = (10.2 eV)\left(Z-1\right)^2</math>, where Z is the atomic number. For example, K-alpha for iron (Z = 26) is calculated in this fashion as 10.2 eV (25)<sup>2</sup> = 6.38 keV energy. For astrophysical purposes, [[Doppler effect|Doppler]] and other effects (such as gravitational broadening) show the iron line to no better accuracy than 6.4 keV. [http://www.journals.uchicago.edu/cgi-bin/resolve?id=doi:10.1086/340992&erFrom=-6703307617883835216Guest] | |||
== Values of Transition Energies == | |||
*Values of different kinds of transition energies like K<sub><math>\alpha</math></sub>, K<sub><math>\beta</math></sub>, L<sub><math>\alpha</math></sub>, L<sub><math>\beta</math></sub> and so on for different elements can be found in the NIST X-Ray Transition Energies Database.<ref>[[NIST]] X-Ray Transition Energies Database [http://physics.nist.gov/PhysRefData/XrayTrans/index.html]</ref> | |||
*K-alpha emission values for hydrogen-like and helium-like ions can be found on Table 1-5 of the LNBL X-Ray Data Booklet <ref>[[Lawrence Berkeley National Laboratory]] X-Ray Data Booklet [http://xdb.lbl.gov/]</ref> | |||
==References== | |||
{{reflist}} | |||
[[Category:Spectroscopy]] | |||
[[Category:X-rays]] | |||
[[Category:Quantum chemistry]] | |||
[[Category:Astronomical spectroscopy]] | |||
[[Category:Atomic physics]] | |||
Latest revision as of 14:37, 26 September 2012
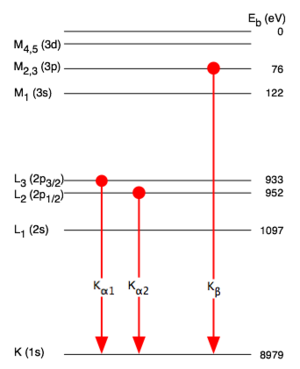
In X-ray spectroscopy, K-alpha emission lines result when an electron transitions to the innermost "K" shell (principal quantum number 1) from a 2p orbital of the second or "L" shell (with principal quantum number 2). The line is actually a doublet, with slightly different energies depending on spin-orbit interaction energy between the electron spin and the orbital momentum of the 2p orbital. K-alpha is typically by far the strongest X-ray spectral line for an element bombarded with energy sufficient to cause maximally intense X-ray emission.
The analogous K-alpha spectra line in hydrogen is known as Lyman alpha; however because of hydrogen's small nuclear charge, this line is in the ultraviolet, not the X-ray range. See Siegbahn notation for the newer IUPAC-recommended spectral notation system.
An example of K-alpha lines are those seen for iron as iron atoms radiating X-rays spiralling into a black hole at the center of a galaxy [1]. For such purposes, the energy of the line is adequately calculated to 2-digit accuracy by the use of Moseley's law: , where Z is the atomic number. For example, K-alpha for iron (Z = 26) is calculated in this fashion as 10.2 eV (25)2 = 6.38 keV energy. For astrophysical purposes, Doppler and other effects (such as gravitational broadening) show the iron line to no better accuracy than 6.4 keV. [2]
Values of Transition Energies
- Values of different kinds of transition energies like K, K, L, L and so on for different elements can be found in the NIST X-Ray Transition Energies Database.[1]
- K-alpha emission values for hydrogen-like and helium-like ions can be found on Table 1-5 of the LNBL X-Ray Data Booklet [2]
References
43 year old Petroleum Engineer Harry from Deep River, usually spends time with hobbies and interests like renting movies, property developers in singapore new condominium and vehicle racing. Constantly enjoys going to destinations like Camino Real de Tierra Adentro.