Income: Difference between revisions
en>DVdm m Reverted edits by 120.56.219.145 (talk) to last revision by ClueBot NG (HG) |
No edit summary |
||
| Line 1: | Line 1: | ||
The '''isoelectric point''' ('''pI'''), sometimes abbreviated to '''IEP''', is the [[pH]] at which a particular [[molecule]] or surface carries no net [[electric charge|electrical charge]]. | |||
[[Amphoteric]] molecules called [[zwitterion]]s contain both positive and negative charges depending on the [[functional groups]] present in the molecule. The net charge on the molecule is affected by pH of its surrounding environment and can become more positively or negatively charged due to the gain or loss, respectively, of [[protons#In Physics and biochemistry|protons]] (H<sup>+</sup>). The pI is the pH value at which the molecule carries no electrical charge or the negative and positive charges are equal. | |||
Surfaces naturally charge to form a [[double layer (interfacial)|double layer]]. In the common case when the surface charge-determining ions are H<sup>+</sup>/OH<sup>-</sup>, the net surface charge is affected by the pH of the liquid in which the solid is submerged. | |||
The pI value can affect the solubility of a molecule at a given pH. Such molecules have minimum [[solubility]] in water or salt solutions at the pH that corresponds to their '''pI''' and often [[precipitate]] out of [[solution]]. Biological amphoteric molecules such as [[protein]]s contain both acidic and basic [[functional groups]]. Amino acids that make up proteins may be positive, negative, neutral, or polar in nature, and together give a protein its overall charge. At a [[pH]] below their pI, proteins carry a net positive charge; above their pI they carry a net negative charge. Proteins can, thus, be separated according to their isoelectric point (overall charge) on a [[polyacrylamide gel]] using a technique called [[isoelectric focusing]], which uses a pH gradient to separate proteins. Isoelectric focusing is also the first step in [[Two-dimensional gel electrophoresis|2-D gel polyacrylamide gel electrophoresis]]. | |||
== Calculating pI values == | |||
For an [[amino acid]] with only one [[amine]] and one [[carboxyl]] group, the pI can be calculated from the [[mean]] of the [[pKa]]s of this molecule.<ref>For derivation of this expression see [[acid dissociation constant#Isoelectric point|acid dissociation constant]]</ref> | |||
: <math> pI = {{pKa_1} + {pKa_2} \over 2} </math> | |||
The [[pH]] of an electrophoretic gel is determined by the [[Buffer solution|buffer]] used for that gel. If the [[pH]] of the buffer is above the pI of the protein being run, the [[protein]] will migrate to the positive pole (negative charge is attracted to a positive pole). If the [[pH]] of the buffer is below the pI of the [[protein]] being run, the [[protein]] will migrate to the negative pole of the gel (positive charge is attracted to the negative pole). If the [[protein]] is run with a buffer pH that is equal to the pI, it will not migrate at all. This is also true for individual amino acids. | |||
=== Examples === | |||
{| | |||
|- | |||
|[[File:Glycine pI.png|250px]] | |||
|[[File:AMP pI.png|250px]] | |||
|- | |||
|align=center|glycine pK = 2.72, 9.60 | |||
|align=center|adenosine monophosphate pK = 2.15, 9.16, 10.67 | |||
|} | |||
In these two examples the isoelectric point is shown by the green vertical line. In [[glycine]] the pK values are separated by nearly 7 units so the concentration of the neutral species, glycine (GlyH), is effectively 100% of the analytical glycine concentration. Glycine may exist as a [[zwitterion]] at the isoelectric point, but the equilibrium constant for the isomerization reaction in solution | |||
:H<sub>2</sub>NCH<sub>2</sub>CO<sub>2</sub>H {{eqm}} H<sub>3</sub>N<sup>+</sup>CH<sub>2</sub>CO<sub>2</sub><sup>-</sup> | |||
is not known. | |||
The other example, [[adenosine monophosphate]] is shown to illustrate the fact that a third species may, in principle, be involved. In fact the concentration of (AMP)H<sub>3</sub><sup>2+</sup> is negligible at the isoelectric point in this case. | |||
If PI is greater than pH, the molecule will positively charge, and reverse is true. | |||
== Ceramic materials == | |||
The isoelectric points (IEP) of metal oxide ceramics are used extensively in material science in various aqueous processing steps (synthesis, modification, etc.). In the absence of chemisorbed or physisorbed species<ref name="ref2pineapple"> | |||
{{cite journal | |||
| last1=Hanaor | |||
| first1=D.A.H. | |||
| last2=Michelazzi | |||
| first2=M. | |||
| last3=Leonelli | |||
| first3=C. | |||
| last4=Sorrell | |||
| first4=C.C. | |||
| title= The effects of carboxylic acids on the aqueous dispersion and electrophoretic deposition of ZrO<sub>2</sub> | |||
| journal= Journal of the European Ceramic Society | |||
| year=2012 | |||
| volume=32 | |||
| issue=1 | |||
| pages=235–244 | |||
| url=http://www.sciencedirect.com/science/article/pii/S0955221911004171 | |||
| doi=10.1016/j.jeurceramsoc.2011.08.015}}</ref> particle surfaces in aqueous suspension are generally assumed to be covered with surface hydroxyl species, M-OH (where M is a metal such as Al, Si, etc.). At pH values above the IEP, the predominate surface species is M-O<sup>-</sup>, while at pH values below the IEP, M-OH<sub>2</sub><sup>+</sup> species predominate. Some approximate values of common ceramics are listed below (Haruta<ref>Haruta M (2004). 'Nanoparticulate Gold Catalysts for Low-Temperature CO Oxidation', ''Journal of New Materials for Electrochemical Systems'', vol. 7, pp 163–172.</ref> and Brunelle,<ref>[http://www.iupac.org/publications/pac/1978/pdf/5009x1211.pdf Brunelle JP (1978). 'Preparation of Catalysts by Metallic Complex Adsorption on Mineral Oxides'. ''Pure and Applied Chemistry'' vol. 50, pp. 1211-1229.]</ref> except where noted). The exact value can vary widely, depending on material factors such as purity and phase as well as physical parameters such as temperature. In addition, precise measurement of isoelectric points is difficult and requires careful techniques, even with modern methods. Thus, many sources often cite differing values for isoelectric points of these materials. | |||
=== Examples of isoelectric points === | |||
The following list gives the pH<sub>25°C</sub> of isoelectric point at 25 °C for selected materials in water: | |||
''Note: The list is ordered by increasing pH values.'' | |||
*[[tungsten(VI) oxide]] WO<sub>3</sub>: 0.2-0.5<ref name="Kosmulski"/> | |||
*[[antimony(V) oxide]] Sb<sub>2</sub>O<sub>5</sub>: <0.4 to 1.9<ref name="Kosmulski"/> | |||
*[[vanadium(V) oxide]] (vanadia) V<sub>2</sub>O<sub>5</sub>: 1-2<ref name="Jolivet"/> (3<ref name="Kosmulski"/>) | |||
*[[silicon dioxide]] (silica) SiO<sub>2</sub>: 1.7-3.5<ref name="Kosmulski"/> | |||
*[[silicon carbide]] (alpha) SiC: 2-3.5<ref>U.S. Patent 5,165,996</ref> | |||
*[[tantalum(V) oxide]], Ta<sub>2</sub>O<sub>5</sub>: 2.7-3.0<ref name="Kosmulski"/> | |||
*[[tin(IV) oxide]] SnO<sub>2</sub>: 4-5.5 (7.3<ref name="Lewis">Lewis, JA (2000). 'Colloidal Processing of Ceramics', ''Journal of the American Ceramic Society'' vol. 83, no. 10, pp.2341–2359.</ref>) | |||
*[[zirconium(IV) oxide]] (zirconia) ZrO<sub>2</sub>: 4-11<ref name="Kosmulski"/> | |||
*[[manganese(IV) oxide]] MnO<sub>2</sub>: 4-5 | |||
*[[indium tin oxide]] ITO: 6<ref>Daido T and Akaike T (1993). 'Electrochemistry of cytochrome c: influence of coulombic attraction with indium tin oxide electrode', ''Journal of Electroanalytical Chemistry'' vol. 344, no. 1-2, pp. 91–106.</ref> | |||
*delta-MnO<sub>2</sub> 1.5, beta-MnO<sub>2</sub> 7.3<ref name="Jolivet"/> | |||
*[[titanium(IV) oxide]] (titania) ([[rutile]] or [[anatase]]) TiO<sub>2</sub>: 3.9-8.2<ref name="Kosmulski"/> | |||
*[[silicon nitride]] Si<sub>3</sub>N<sub>4</sub>: 6-7 | |||
*[[magnetite|iron (II, III) oxide]] (magnetite) Fe<sub>3</sub>O<sub>4</sub>: 6.5-6.8<ref name="Kosmulski"/> | |||
*[[maghemite|gamma iron (III) oxide]] (maghemite) Fe<sub>2</sub>O<sub>3</sub>: 3.3-6.7<ref name="Kosmulski"/> | |||
*[[cerium(IV) oxide]] (ceria) CeO<sub>2</sub>: 6.7-8.6<ref name="Kosmulski"/> | |||
*[[chromium(III) oxide]] (chromia) Cr<sub>2</sub>O<sub>3</sub>: 7<ref name="Jolivet"/> (6.2-8.1<ref name="Kosmulski"/>) | |||
*gamma [[aluminium oxide]] (gamma alumina) Al<sub>2</sub>O<sub>3</sub>: 7-8 | |||
*[[thallium(I) oxide]] Tl<sub>2</sub>O: 8<ref>Kosmulski M and Saneluta C (2004). 'Point of zero charge/isoelectric point of exotic oxides: Tl2O3', ''Journal of Colloid and Interface Science'' vol. 280, no. 2, pp. 544–545.</ref> | |||
*[[hematite|alpha iron (III) oxide]] (hematite) Fe<sub>2</sub>O<sub>3</sub>: 8.4-8.5<ref name="Kosmulski"/> | |||
*alpha [[aluminium oxide]] (alpha alumina, corundum) Al<sub>2</sub>O<sub>3</sub>: 8-9 | |||
*[[silicon nitride]] Si<sub>3</sub>N<sub>4</sub>: 9<ref name="Lewis"/> | |||
*[[yttrium(III) oxide]] (yttria) Y<sub>2</sub>O<sub>3</sub>: 7.15-8.95<ref name="Kosmulski"/> | |||
*[[copper(II) oxide]] CuO: 9.5<ref name="Lewis"/> | |||
*[[zinc oxide]] ZnO: 8.7-10.3<ref name="Kosmulski"/> | |||
*[[lanthanum(III) oxide]] La<sub>2</sub>O<sub>3</sub>: 10 | |||
*[[nickel(II) oxide]] NiO: 10-11<ref name="Lewis"/> (9.9-11.3<ref name="Kosmulski"/>) | |||
*[[lead(II) oxide]] PbO: 10.7-11.6<ref name="Kosmulski">Marek Kosmulski, "Chemical Properties of Material Surfaces", Marcel Dekker, 2001.</ref> | |||
*[[magnesium oxide]] (magnesia) MgO: 12-13 (9.8-12.7<ref name="Kosmulski"/>) | |||
Mixed oxides may exhibit isoelectric point values that are intermediate to those of the corresponding pure oxides. For example, Jara ''et al.''<ref>Jara, A.A., S. Goldberg and M.L. Mora (2005). 'Studies of the surface charge of amorphous aluminosilicates using surface complexation models', ''Journal of Colloid and Interface Science'', vol. 292, no. 1, pp. 160–170.</ref> measured an IEP of 4.5 for a synthetically prepared amorphous [[aluminosilicate]] (Al<sub>2</sub>O<sub>3</sub>-SiO<sub>2</sub>). The researchers noted that the electrokinetic behavior of the surface was dominated by surface Si-OH species, thus explaining the relatively low IEP value. Significantly higher IEP values (pH 6 to 8) have been reported for 3Al<sub>2</sub>O<sub>3</sub>-2SiO<sub>2</sub> by others (see Lewis<ref name="Lewis"/>). Lewis<ref name="Lewis"/> also lists the IEP of [[barium titanate]], BaTiO<sub>3</sub> as being between pH 5 and 6, while Vamvakaki et al.<ref>[http://www.rsc.org/ej/JM/2001/b101728o.pdf Vamvakaki, M., N.C. Billingham, S.P. Armes, J.F. Watts and S.J. Greaves (2001). 'Controlled structure copolymers for the dispersion of high-performance ceramics in aqueous media', ''Journal of Materials Chemistry'', vol. 11, pp. 2437-2444.]</ref> reported a value of 3, although these authors note that a wide range of values have been reported, a result of either residual [[barium carbonate]] on the surface or TiO<sub>2</sub>-rich surfaces. | |||
The farther the pH of an Amino Acid solution is from its pl the greater the electric charge on that population of molecules. | |||
== Isoelectric point versus point of zero charge == | |||
The terms isoelectric point (IEP) and [[point of zero charge]] (PZC) are often used interchangeably, although under certain circumstances, it may be productive to make the distinction. | |||
In systems in which H<sup>+</sup>/OH<sup>-</sup> are the interface potential-determining ions, the point of zero charge is given in terms of pH. The pH at which the surface exhibits a neutral net electrical charge is the point of zero charge at the surface. [[Electrokinetic phenomena]] generally measure [[zeta potential]], and a zero zeta potential is interpreted as the point of zero net charge at the [[Electrical double layer|shear plane]]. This is termed the isoelectric point.<ref>A.W. Adamson, A.P. Gast, "Physical Chemistry of Surfaces", John Wiley and Sons, 1997.</ref> Thus, the isoelectric point is the value of pH at which the colloidal particle remains stationary in an electrical field. The isoelectric point is expected to be somewhat different than the point of zero charge at the particle surface, but this difference is often ignored in practice for so-called pristine surfaces, i.e., surfaces with no [[adsorption|specifically adsorbed]] positive or negative charges.<ref name="ref2pineapple" /> In this context, specific adsorption is understood as adsorption occurring in a [[Double layer (interfacial)|Stern layer]] or [[chemisorption]]. Thus, point of zero charge at the surface is taken as equal to isoelectric point in the absence of specific adsorption on that surface. | |||
According to Jolivet,<ref name="Jolivet">Jolivet J.P., ''Metal Oxide Chemistry and Synthesis. From Solution to Solid State'', John Wiley & Sons Ltd. 2000, ISBN 0-471-97056-5 (English translation of the original French text, ''De la Solution à l'Oxyde'', InterEditions et CNRS Editions, Paris, 1994).</ref> in the absence of positive or negative charges, the surface is best described by the point of zero charge. If positive and negative charges are both present in equal amounts, then this is the isoelectric point. Thus, the PZC refers to the absence of any type of surface charge, while the IEP refers to a state of neutral net surface charge. The difference between the two, therefore, is the quantity of charged sites at the point of net zero charge. Jolivet uses the intrinsic surface equilibrium constants, pK<sup>-</sup> and pK<sup>+</sup> to define the two conditions in terms of the relative number of charged sites: | |||
:<math> pK^- - pK^+ = \Delta pK = \log {\frac{\left[MOH\right]^2}{\left[MOH{_2^+}\right]\left[MO^-\right]}} </math> | |||
For large ΔpK (>4 according to Jolivet), the predominate species is MOH while there are relatively few charged species - so the PZC is relevant. For small values of ΔpK, there are many charged species in approximately equal numbers, so one speaks of the IEP. | |||
== See also == | |||
* [[Isoionic point]] | |||
== References == | |||
{{reflist}} | |||
== Further reading== | |||
* Nelson DL, Cox MM (2004). ''Lehninger Principles of Biochemistry''. W. H. Freeman; 4th edition (Hardcover). ISBN 0-7167-4339- | |||
* Kosmulski M. (2009). ''Surface Charging and Points of Zero Charge''. CRC Press; 1st edition (Hardcover). ISBN 978-1-4200-5188-9 | |||
== External links == | |||
* [http://www.embl-heidelberg.de/cgi/pi-wrapper.pl EMBL WWW Gateway to Isoelectric Point Service] — calculates the pI for an input amino acid sequence. | |||
* [http://isoelectric.ovh.org Calculation of protein isoelectric point] — free online and offline program to calculation pI and more theoretical information about this subject. | |||
* Isoelectric point determination and Charge versus pH plot of amphoteric molecules (e.g., amino acids) by a [http://www2.iq.usp.br/docente/gutz/Curtipot_.html free suite of spreadsheets for computing acid-base equilibria.] | |||
{{DEFAULTSORT:Isoelectric Point}} | |||
[[Category:Ions]] | |||
[[Category:Molecular biology]] | |||
Revision as of 21:45, 5 September 2013
The isoelectric point (pI), sometimes abbreviated to IEP, is the pH at which a particular molecule or surface carries no net electrical charge.
Amphoteric molecules called zwitterions contain both positive and negative charges depending on the functional groups present in the molecule. The net charge on the molecule is affected by pH of its surrounding environment and can become more positively or negatively charged due to the gain or loss, respectively, of protons (H+). The pI is the pH value at which the molecule carries no electrical charge or the negative and positive charges are equal.
Surfaces naturally charge to form a double layer. In the common case when the surface charge-determining ions are H+/OH-, the net surface charge is affected by the pH of the liquid in which the solid is submerged.
The pI value can affect the solubility of a molecule at a given pH. Such molecules have minimum solubility in water or salt solutions at the pH that corresponds to their pI and often precipitate out of solution. Biological amphoteric molecules such as proteins contain both acidic and basic functional groups. Amino acids that make up proteins may be positive, negative, neutral, or polar in nature, and together give a protein its overall charge. At a pH below their pI, proteins carry a net positive charge; above their pI they carry a net negative charge. Proteins can, thus, be separated according to their isoelectric point (overall charge) on a polyacrylamide gel using a technique called isoelectric focusing, which uses a pH gradient to separate proteins. Isoelectric focusing is also the first step in 2-D gel polyacrylamide gel electrophoresis.
Calculating pI values
For an amino acid with only one amine and one carboxyl group, the pI can be calculated from the mean of the pKas of this molecule.[1]
The pH of an electrophoretic gel is determined by the buffer used for that gel. If the pH of the buffer is above the pI of the protein being run, the protein will migrate to the positive pole (negative charge is attracted to a positive pole). If the pH of the buffer is below the pI of the protein being run, the protein will migrate to the negative pole of the gel (positive charge is attracted to the negative pole). If the protein is run with a buffer pH that is equal to the pI, it will not migrate at all. This is also true for individual amino acids.
Examples

|
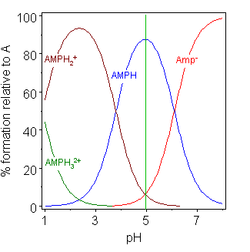
|
| glycine pK = 2.72, 9.60 | adenosine monophosphate pK = 2.15, 9.16, 10.67 |
In these two examples the isoelectric point is shown by the green vertical line. In glycine the pK values are separated by nearly 7 units so the concentration of the neutral species, glycine (GlyH), is effectively 100% of the analytical glycine concentration. Glycine may exist as a zwitterion at the isoelectric point, but the equilibrium constant for the isomerization reaction in solution
- H2NCH2CO2H Template:Eqm H3N+CH2CO2-
is not known.
The other example, adenosine monophosphate is shown to illustrate the fact that a third species may, in principle, be involved. In fact the concentration of (AMP)H32+ is negligible at the isoelectric point in this case. If PI is greater than pH, the molecule will positively charge, and reverse is true.
Ceramic materials
The isoelectric points (IEP) of metal oxide ceramics are used extensively in material science in various aqueous processing steps (synthesis, modification, etc.). In the absence of chemisorbed or physisorbed species[2] particle surfaces in aqueous suspension are generally assumed to be covered with surface hydroxyl species, M-OH (where M is a metal such as Al, Si, etc.). At pH values above the IEP, the predominate surface species is M-O-, while at pH values below the IEP, M-OH2+ species predominate. Some approximate values of common ceramics are listed below (Haruta[3] and Brunelle,[4] except where noted). The exact value can vary widely, depending on material factors such as purity and phase as well as physical parameters such as temperature. In addition, precise measurement of isoelectric points is difficult and requires careful techniques, even with modern methods. Thus, many sources often cite differing values for isoelectric points of these materials.
Examples of isoelectric points
The following list gives the pH25°C of isoelectric point at 25 °C for selected materials in water:
Note: The list is ordered by increasing pH values.
- tungsten(VI) oxide WO3: 0.2-0.5[5]
- antimony(V) oxide Sb2O5: <0.4 to 1.9[5]
- vanadium(V) oxide (vanadia) V2O5: 1-2[6] (3[5])
- silicon dioxide (silica) SiO2: 1.7-3.5[5]
- silicon carbide (alpha) SiC: 2-3.5[7]
- tantalum(V) oxide, Ta2O5: 2.7-3.0[5]
- tin(IV) oxide SnO2: 4-5.5 (7.3[8])
- zirconium(IV) oxide (zirconia) ZrO2: 4-11[5]
- manganese(IV) oxide MnO2: 4-5
- indium tin oxide ITO: 6[9]
- delta-MnO2 1.5, beta-MnO2 7.3[6]
- titanium(IV) oxide (titania) (rutile or anatase) TiO2: 3.9-8.2[5]
- silicon nitride Si3N4: 6-7
- iron (II, III) oxide (magnetite) Fe3O4: 6.5-6.8[5]
- gamma iron (III) oxide (maghemite) Fe2O3: 3.3-6.7[5]
- cerium(IV) oxide (ceria) CeO2: 6.7-8.6[5]
- chromium(III) oxide (chromia) Cr2O3: 7[6] (6.2-8.1[5])
- gamma aluminium oxide (gamma alumina) Al2O3: 7-8
- thallium(I) oxide Tl2O: 8[10]
- alpha iron (III) oxide (hematite) Fe2O3: 8.4-8.5[5]
- alpha aluminium oxide (alpha alumina, corundum) Al2O3: 8-9
- silicon nitride Si3N4: 9[8]
- yttrium(III) oxide (yttria) Y2O3: 7.15-8.95[5]
- copper(II) oxide CuO: 9.5[8]
- zinc oxide ZnO: 8.7-10.3[5]
- lanthanum(III) oxide La2O3: 10
- nickel(II) oxide NiO: 10-11[8] (9.9-11.3[5])
- lead(II) oxide PbO: 10.7-11.6[5]
- magnesium oxide (magnesia) MgO: 12-13 (9.8-12.7[5])
Mixed oxides may exhibit isoelectric point values that are intermediate to those of the corresponding pure oxides. For example, Jara et al.[11] measured an IEP of 4.5 for a synthetically prepared amorphous aluminosilicate (Al2O3-SiO2). The researchers noted that the electrokinetic behavior of the surface was dominated by surface Si-OH species, thus explaining the relatively low IEP value. Significantly higher IEP values (pH 6 to 8) have been reported for 3Al2O3-2SiO2 by others (see Lewis[8]). Lewis[8] also lists the IEP of barium titanate, BaTiO3 as being between pH 5 and 6, while Vamvakaki et al.[12] reported a value of 3, although these authors note that a wide range of values have been reported, a result of either residual barium carbonate on the surface or TiO2-rich surfaces.
The farther the pH of an Amino Acid solution is from its pl the greater the electric charge on that population of molecules.
Isoelectric point versus point of zero charge
The terms isoelectric point (IEP) and point of zero charge (PZC) are often used interchangeably, although under certain circumstances, it may be productive to make the distinction.
In systems in which H+/OH- are the interface potential-determining ions, the point of zero charge is given in terms of pH. The pH at which the surface exhibits a neutral net electrical charge is the point of zero charge at the surface. Electrokinetic phenomena generally measure zeta potential, and a zero zeta potential is interpreted as the point of zero net charge at the shear plane. This is termed the isoelectric point.[13] Thus, the isoelectric point is the value of pH at which the colloidal particle remains stationary in an electrical field. The isoelectric point is expected to be somewhat different than the point of zero charge at the particle surface, but this difference is often ignored in practice for so-called pristine surfaces, i.e., surfaces with no specifically adsorbed positive or negative charges.[2] In this context, specific adsorption is understood as adsorption occurring in a Stern layer or chemisorption. Thus, point of zero charge at the surface is taken as equal to isoelectric point in the absence of specific adsorption on that surface.
According to Jolivet,[6] in the absence of positive or negative charges, the surface is best described by the point of zero charge. If positive and negative charges are both present in equal amounts, then this is the isoelectric point. Thus, the PZC refers to the absence of any type of surface charge, while the IEP refers to a state of neutral net surface charge. The difference between the two, therefore, is the quantity of charged sites at the point of net zero charge. Jolivet uses the intrinsic surface equilibrium constants, pK- and pK+ to define the two conditions in terms of the relative number of charged sites:
For large ΔpK (>4 according to Jolivet), the predominate species is MOH while there are relatively few charged species - so the PZC is relevant. For small values of ΔpK, there are many charged species in approximately equal numbers, so one speaks of the IEP.
See also
References
43 year old Petroleum Engineer Harry from Deep River, usually spends time with hobbies and interests like renting movies, property developers in singapore new condominium and vehicle racing. Constantly enjoys going to destinations like Camino Real de Tierra Adentro.
Further reading
- Nelson DL, Cox MM (2004). Lehninger Principles of Biochemistry. W. H. Freeman; 4th edition (Hardcover). ISBN 0-7167-4339-
- Kosmulski M. (2009). Surface Charging and Points of Zero Charge. CRC Press; 1st edition (Hardcover). ISBN 978-1-4200-5188-9
External links
- EMBL WWW Gateway to Isoelectric Point Service — calculates the pI for an input amino acid sequence.
- Calculation of protein isoelectric point — free online and offline program to calculation pI and more theoretical information about this subject.
- Isoelectric point determination and Charge versus pH plot of amphoteric molecules (e.g., amino acids) by a free suite of spreadsheets for computing acid-base equilibria.
- ↑ For derivation of this expression see acid dissociation constant
- ↑ 2.0 2.1
One of the biggest reasons investing in a Singapore new launch is an effective things is as a result of it is doable to be lent massive quantities of money at very low interest rates that you should utilize to purchase it. Then, if property values continue to go up, then you'll get a really high return on funding (ROI). Simply make sure you purchase one of the higher properties, reminiscent of the ones at Fernvale the Riverbank or any Singapore landed property Get Earnings by means of Renting
In its statement, the singapore property listing - website link, government claimed that the majority citizens buying their first residence won't be hurt by the new measures. Some concessions can even be prolonged to chose teams of consumers, similar to married couples with a minimum of one Singaporean partner who are purchasing their second property so long as they intend to promote their first residential property. Lower the LTV limit on housing loans granted by monetary establishments regulated by MAS from 70% to 60% for property purchasers who are individuals with a number of outstanding housing loans on the time of the brand new housing purchase. Singapore Property Measures - 30 August 2010 The most popular seek for the number of bedrooms in Singapore is 4, followed by 2 and three. Lush Acres EC @ Sengkang
Discover out more about real estate funding in the area, together with info on international funding incentives and property possession. Many Singaporeans have been investing in property across the causeway in recent years, attracted by comparatively low prices. However, those who need to exit their investments quickly are likely to face significant challenges when trying to sell their property – and could finally be stuck with a property they can't sell. Career improvement programmes, in-house valuation, auctions and administrative help, venture advertising and marketing, skilled talks and traisning are continuously planned for the sales associates to help them obtain better outcomes for his or her shoppers while at Knight Frank Singapore. No change Present Rules
Extending the tax exemption would help. The exemption, which may be as a lot as $2 million per family, covers individuals who negotiate a principal reduction on their existing mortgage, sell their house short (i.e., for lower than the excellent loans), or take part in a foreclosure course of. An extension of theexemption would seem like a common-sense means to assist stabilize the housing market, but the political turmoil around the fiscal-cliff negotiations means widespread sense could not win out. Home Minority Chief Nancy Pelosi (D-Calif.) believes that the mortgage relief provision will be on the table during the grand-cut price talks, in response to communications director Nadeam Elshami. Buying or promoting of blue mild bulbs is unlawful.
A vendor's stamp duty has been launched on industrial property for the primary time, at rates ranging from 5 per cent to 15 per cent. The Authorities might be trying to reassure the market that they aren't in opposition to foreigners and PRs investing in Singapore's property market. They imposed these measures because of extenuating components available in the market." The sale of new dual-key EC models will even be restricted to multi-generational households only. The models have two separate entrances, permitting grandparents, for example, to dwell separately. The vendor's stamp obligation takes effect right this moment and applies to industrial property and plots which might be offered inside three years of the date of buy. JLL named Best Performing Property Brand for second year running
The data offered is for normal info purposes only and isn't supposed to be personalised investment or monetary advice. Motley Fool Singapore contributor Stanley Lim would not personal shares in any corporations talked about. Singapore private home costs increased by 1.eight% within the fourth quarter of 2012, up from 0.6% within the earlier quarter. Resale prices of government-built HDB residences which are usually bought by Singaporeans, elevated by 2.5%, quarter on quarter, the quickest acquire in five quarters. And industrial property, prices are actually double the levels of three years ago. No withholding tax in the event you sell your property. All your local information regarding vital HDB policies, condominium launches, land growth, commercial property and more
There are various methods to go about discovering the precise property. Some local newspapers (together with the Straits Instances ) have categorised property sections and many local property brokers have websites. Now there are some specifics to consider when buying a 'new launch' rental. Intended use of the unit Every sale begins with 10 p.c low cost for finish of season sale; changes to 20 % discount storewide; follows by additional reduction of fiftyand ends with last discount of 70 % or extra. Typically there is even a warehouse sale or transferring out sale with huge mark-down of costs for stock clearance. Deborah Regulation from Expat Realtor shares her property market update, plus prime rental residences and houses at the moment available to lease Esparina EC @ Sengkang - ↑ Haruta M (2004). 'Nanoparticulate Gold Catalysts for Low-Temperature CO Oxidation', Journal of New Materials for Electrochemical Systems, vol. 7, pp 163–172.
- ↑ Brunelle JP (1978). 'Preparation of Catalysts by Metallic Complex Adsorption on Mineral Oxides'. Pure and Applied Chemistry vol. 50, pp. 1211-1229.
- ↑ 5.00 5.01 5.02 5.03 5.04 5.05 5.06 5.07 5.08 5.09 5.10 5.11 5.12 5.13 5.14 5.15 5.16 Marek Kosmulski, "Chemical Properties of Material Surfaces", Marcel Dekker, 2001.
- ↑ 6.0 6.1 6.2 6.3 Jolivet J.P., Metal Oxide Chemistry and Synthesis. From Solution to Solid State, John Wiley & Sons Ltd. 2000, ISBN 0-471-97056-5 (English translation of the original French text, De la Solution à l'Oxyde, InterEditions et CNRS Editions, Paris, 1994).
- ↑ U.S. Patent 5,165,996
- ↑ 8.0 8.1 8.2 8.3 8.4 8.5 Lewis, JA (2000). 'Colloidal Processing of Ceramics', Journal of the American Ceramic Society vol. 83, no. 10, pp.2341–2359.
- ↑ Daido T and Akaike T (1993). 'Electrochemistry of cytochrome c: influence of coulombic attraction with indium tin oxide electrode', Journal of Electroanalytical Chemistry vol. 344, no. 1-2, pp. 91–106.
- ↑ Kosmulski M and Saneluta C (2004). 'Point of zero charge/isoelectric point of exotic oxides: Tl2O3', Journal of Colloid and Interface Science vol. 280, no. 2, pp. 544–545.
- ↑ Jara, A.A., S. Goldberg and M.L. Mora (2005). 'Studies of the surface charge of amorphous aluminosilicates using surface complexation models', Journal of Colloid and Interface Science, vol. 292, no. 1, pp. 160–170.
- ↑ Vamvakaki, M., N.C. Billingham, S.P. Armes, J.F. Watts and S.J. Greaves (2001). 'Controlled structure copolymers for the dispersion of high-performance ceramics in aqueous media', Journal of Materials Chemistry, vol. 11, pp. 2437-2444.
- ↑ A.W. Adamson, A.P. Gast, "Physical Chemistry of Surfaces", John Wiley and Sons, 1997.

![{\displaystyle pK^{-}-pK^{+}=\Delta pK=\log {\frac {\left[MOH\right]^{2}}{\left[MOH{_{2}^{+}}\right]\left[MO^{-}\right]}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/fe218700836c34df9f64f9069668d11ed4f1a741)