Carrier generation and recombination: Difference between revisions
No edit summary |
en>FrescoBot m Bot: standard sections headers and minor changes |
||
| Line 1: | Line 1: | ||
{{Use dmy dates|date=July 2013}} | |||
{{Chembox | |||
| Watchedfields = changed | |||
| verifiedrevid = 477167117 | |||
| ImageFileL1 = Ethanol-2D-flat.png | |||
| ImageFileL1_Ref = {{chemboximage|correct|??}} | |||
| ImageSizeL1 = 131 | |||
| ImageNameL1 = Full structural formula of ethanol | |||
| ImageFileR1 = Ethanol-2D-skeletal.svg | |||
| ImageFileR1_Ref = {{chemboximage|correct|??}} | |||
| ImageSizeR1 = 111 | |||
| ImageNameR1 = Skeletal formula of ethanol | |||
| ImageFileL2 = Ethanol-3D-balls.png | |||
| ImageFileL2_Ref = {{chemboximage|correct|??}} | |||
| ImageSizeL2 = 131 | |||
| ImageNameL2 = Ball-and-stick model of ethanol | |||
| ImageFileR2 = Ethanol-3D-vdW.png | |||
| ImageFileR2_Ref = {{chemboximage|correct|??}} | |||
| ImageSizeR2 = 111 | |||
| ImageNameR2 = Space-filling model of ethanol | |||
| SystematicName = Ethanol<ref name="Pubchem">{{cite web|title = Ethanol – Compound Summary|url = http://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?cid=702|work = The PubChem Project|location = USA|publisher = National Center for Biotechnology Information}}</ref> | |||
| OtherNames = {{Plainlist| | |||
* Absolute alcohol | |||
* Alcohol | |||
* Drinking alcohol | |||
* Ethyl alcohol | |||
* Ethyl hydrate | |||
* Ethyl hydroxide | |||
* Ethylic alcohol | |||
* Ethylol | |||
* Grain alcohol | |||
* Hydroxyethane | |||
* Methylcarbinol | |||
}} | |||
| Section1 = {{Chembox Identifiers | |||
| CASNo = 64-17-5 | |||
| CASNo_Ref = {{cascite|correct|CAS}} | |||
| PubChem = 702 | |||
| PubChem_Ref = {{Pubchemcite|correct|PubChem}} | |||
| ChemSpiderID = 682 | |||
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | |||
| UNII = 3K9958V90M | |||
| UNII_Ref = {{fdacite|correct|FDA}} | |||
| EINECS = 200-578-6 | |||
| UNNumber = 1170 | |||
| DrugBank_Ref = {{drugbankcite|correct|drugbank}} | |||
| DrugBank = DB00898 | |||
| KEGG = D00068 | |||
| KEGG_Ref = {{keggcite|correct|kegg}} | |||
| MeSHName = Ethanol | |||
| ChEBI_Ref = {{ebicite|correct|EBI}} | |||
| ChEBI = 16236 | |||
| ChEMBL = 545 | |||
| ChEMBL_Ref = {{ebicite|correct|EBI}} | |||
| RTECS = KQ6300000 | |||
| ATCCode_prefix = D01 | |||
| ATCCode_suffix = AE06 | |||
| ATC_Supplemental = {{ATC|D08|AX08}}, {{ATC|V03|AB16}}, {{ATC|V03|AZ01}} | |||
| Beilstein = 1718733 | |||
| Gmelin = 787 | |||
| 3DMet = B01253 | |||
| SMILES = CCO | |||
| StdInChI = 1S/C2H6O/c1-2-3/h3H,2H2,1H3 | |||
| StdInChI_Ref = {{stdinchicite|correct|chemspider}} | |||
| InChI = 1/C2H6O/c1-2-3/h3H,2H2,1H3 | |||
| StdInChIKey = LFQSCWFLJHTTHZ-UHFFFAOYSA-N | |||
| StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | |||
| InChIKey = LFQSCWFLJHTTHZ-UHFFFAOYAB}} | |||
| Section2 = {{Chembox Properties | |||
| C = 2 | |||
| H = 6 | |||
| O = 1 | |||
| ExactMass = 46.041864814 g mol<sup>−1</sup> | |||
| Appearance = Colorless liquid | |||
| Density = 0.789 g/cm<sup>3</sup> (at 20°C) | |||
| MeltingPtC = −114 | |||
| BoilingPtC = 78.37 | |||
| LogP = -0.18 | |||
| VaporPressure = 5.95 kPa (at 20 °C) | |||
| pKa = 15.9<ref>{{cite journal|doi=10.1021/ja01489a008|year=1960|last1=Ballinger|first1=P.|last2=Long|first2=F. A.|journal=Journal of the American Chemical Society|volume=82|issue=4|page=795}}</ref> | |||
| pKb = -1.9 | |||
| RefractIndex = 1.361 | |||
| Viscosity = 0.0012 Pa s (at 20 °C), 0.001074 Pa s (at 25 °C)<ref name=crc92>{{Cite book|editor=Lide, David R.|title=CRC Handbook of Chemistry and Physics|edition=92|year=2012|publisher=CRC Press/Taylor and Francis|location=Boca Raton, FL.|pages=6–232}}</ref> | |||
| Dipole = 1.69 D<ref name=crc89>{{Cite book|editor=Lide, David R.|title=CRC Handbook of Chemistry and Physics|edition=89|year=2008|publisher=CRC Press|location=Boca Raton|pages=9–55}}</ref> | |||
}} | |||
| Section3 = {{Chembox Pharmacology | |||
| AdminRoutes = Intramuscular<br /> | |||
Intravenous<br /> | |||
Oral<br /> | |||
Topical | |||
| Metabolism = Hepatic | |||
| [[Dependence liability]] – low-moderate | |||
}} | |||
| Section4 = {{Chembox Hazards | |||
| EUIndex = 603-002-00-5 | |||
| EUClass = {{Hazchem F}} | |||
| RPhrases = {{R11}} | |||
| SPhrases = {{S2}}, {{S7}}, {{S16}} | |||
| NFPA-H = 2 | |||
| NFPA-F = 3 | |||
| NFPA-R = 0 | |||
| FlashPt = 13–14 °C | |||
| Autoignition = 363 °C<ref>{{cite web |url=http://www.engineeringtoolbox.com/fuels-ignition-temperatures-d_171.html |title=Fuels and Chemicals – Autoignition Temperatures |publisher=engineeringtoolbox.com |postscript=<!-- Bot inserted parameter. Either remove it; or change its value to "." for the cite to end in a ".", as necessary. -->{{inconsistent citations}} | |||
}}</ref> | |||
| LD50 = 5628 mg kg<sup>−1</sup> (oral, rat) | |||
}} | |||
}} | |||
This page provides supplementary chemical data on [[ethanol]]. Except where noted otherwise, data relate to [[standard ambient temperature and pressure]]. | |||
== Material Safety Data Sheet == <!-- KEEP this header, it is linked to from the infobox on the main article page --> | |||
The handling of this chemical may incur notable safety precautions. It is highly recommend that you seek the Material Safety Datasheet ([[Material safety data sheet|MSDS]]) for this chemical from a reliable source and follow its directions. | |||
== Structure and properties == <!-- KEEP this header, it is linked to from the infobox on the main article page --> | |||
{| border="1" cellspacing="0" cellpadding="3" style="margin: 0 0 0 0.5em; background: #FFFFFF; border-collapse: collapse; border-color: #C0C090;" | |||
! {{chembox header}} | Structure and properties | |||
|- | |||
| [[Index of refraction]], ''n''<sub>25</sub> | |||
| 1.361 <!-- Please omit if not applicable --> | |||
|- | |||
| [[Dielectric constant]], ε<sub>r</sub> | |||
| 24.3 ε<sub>0</sub> at 25 °C <!-- Please omit if not applicable --> | |||
|- | |||
| [[Bond strength (chemistry)|Bond strength]] | |||
| ? <!-- Specify which bond. Please omit if not applicable --> | |||
|- | |||
| [[Bond length]] | |||
| ? <!-- Specify which bond. Please omit if not applicable --> | |||
|- | |||
| [[Bond angle]] | |||
| ? <!-- Specify which angle, e.g. Cl-P-O. Please omit if not applicable --> | |||
|- | |||
| [[Magnetic susceptibility]]<ref>[http://nmr.chem.umn.edu/ResReports/NMR002.html NMR-002: Sample Devices and Magnetic Susceptibility<!-- Bot generated title -->]</ref> | |||
| 5.8·10<sup>−7</sup> ([[cgs]] units, volume) | |||
|- | |||
| [[Surface tension]] | |||
| 22.39 dyn/cm at 25 °C | |||
|- | |||
| [[Viscosity]]<ref name="cheric_p">{{Cite web|url=http://www.cheric.org/research/kdb/hcprop/cmpsrch.php|title=Pure Component Properties|format=Queriable database|publisher=Chemical Engineering Research Information Center|accessdate=12 May 2007}}</ref> | |||
| | |||
{| | |||
|- | |||
| 6.285 mPa·s || at –50 °C | |||
|- | |||
| 4.656 mPa·s || at –40 °C | |||
|- | |||
| 3.530 mPa·s || at –30 °C | |||
|- | |||
| 2.731 mPa·s || at –20 °C | |||
|- | |||
| 2.419 mPa·s || at –15 °C | |||
|- | |||
| 2.151 mPa·s || at –10 °C | |||
|- | |||
| 1.920 mPa·s || at –5 °C | |||
|- | |||
| 1.720 mPa·s || at 0 °C | |||
|- | |||
| 1.546 mPa·s || at 5 °C | |||
|- | |||
| 1.394 mPa·s || at 10 °C | |||
|- | |||
| 1.261 mPa·s || at 15 °C | |||
|- | |||
| 1.144 mPa·s || at 20 °C | |||
|- | |||
| 1.040 mPa·s || at 25 °C | |||
|- | |||
| 0.949 mPa·s || at 30 °C | |||
|- | |||
| 0.794 mPa·s || at 40 °C | |||
|- | |||
| 0.670 mPa·s || at 50 °C | |||
|- | |||
| 0.570 mPa·s || at 60 °C | |||
|- | |||
| 0.487 mPa·s || at 70 °C | |||
|- | |||
| 0.452 mPa·s || at 75 °C | |||
|- | |||
|} | |||
|} | |||
== Thermodynamic properties == <!-- KEEP this header, it is linked to from the infobox on the main article page --> | |||
{| border="1" cellspacing="0" cellpadding="6" style="margin: 0 0 0 0.5em; background: #FFFFFF; border-collapse: collapse; border-color: #C0C090;" | |||
! {{chembox header}} | Phase behavior | |||
|- | |||
| [[Triple point]] | |||
| 150 K (−123 °C), 0.00043 Pa | |||
|- | |||
| [[Critical point (thermodynamics)|Critical point]] | |||
| 514 K (241 °C), 63 bar | |||
|- | |||
| [[Standard enthalpy change of fusion|Std enthalpy change<br/>of fusion]], Δ<sub>fus</sub>''H''<sup><s>o</s></sup> | |||
| +4.9 kJ/mol | |||
|- | |||
| [[Standard entropy change of fusion|Std entropy change<br/>of fusion]], Δ<sub>fus</sub>''S''<sup><s>o</s></sup> | |||
| +31 J/(mol·K) | |||
|- | |||
| [[Standard enthalpy change of vaporization|Std enthalpy change<br/>of vaporization]], Δ<sub>vap</sub>''H''<sup><s>o</s></sup> | |||
| +38.56 kJ/mol | |||
|- | |||
| [[Standard entropy change of vaporization|Std entropy change<br/>of vaporization]], Δ<sub>vap</sub>''S''<sup><s>o</s></sup> | |||
| 109.67 J/(mol·K) | |||
|- | |||
| [[Freezing-point depression|Molal freezing point constant]] | |||
| –1.99 °C kg/mol | |||
|- | |||
| [[Boiling-point elevation|Molal boiling point constant]] | |||
| 1.19 °C kg/mol | |||
|- | |||
! {{chembox header}} | Solid properties | |||
|- | |||
| [[Standard enthalpy change of formation|Std enthalpy change<br/>of formation]], Δ<sub>f</sub>''H''<sup><s>o</s></sup><sub>solid</sub> | |||
| –277.7 kJ/mol | |||
|- | |||
| [[Standard molar entropy]],<br/>''S''<sup><s>o</s></sup><sub>solid</sub> | |||
| 160.7 J/(mol K)<ref name=atkins>{{cite book|last=Atkins|first=Peter|title=Atkins' Physical Chemistry|year=2010|publisher=Oxford University Press|pages=913–947}}</ref> | |||
|- | |||
| [[Heat capacity]], ''c<sub>p</sub>'' | |||
| 111.46 J/(mol K) <ref name="atkins"/> | |||
|- | |||
! {{chembox header}} | Liquid properties | |||
|- | |||
| [[Standard enthalpy change of formation|Std enthalpy change<br/>of formation]], Δ<sub>f</sub>''H''<sup><s>o</s></sup><sub>liquid</sub> | |||
| −277.38 kJ/mol | |||
|- | |||
| [[Standard molar entropy]],<br/>''S''<sup><s>o</s></sup><sub>liquid</sub> | |||
| 159.9 J/(mol K) | |||
|- | |||
| [[Enthalpy of combustion]], Δ<sub>c</sub>''H''<sup><s>o</s></sup> | |||
| –1370.7 kJ/mol | |||
|- | |||
| [[Heat capacity]], ''c<sub>p</sub>'' | |||
| 112.4 J/(mol K) | |||
|- | |||
! {{chembox header}} | Gas properties | |||
|- | |||
| [[Standard enthalpy change of formation|Std enthalpy change<br/>of formation]], Δ<sub>f</sub>''H''<sup><s>o</s></sup><sub>gas</sub> | |||
| −235.3 kJ/mol | |||
|- | |||
| [[Standard molar entropy]],<br/>''S''<sup><s>o</s></sup><sub>gas</sub> | |||
| 283 J/(mol K) | |||
|- | |||
| [[Heat capacity]],<ref name="lange1525">''Lange's Handbook of Chemistry'' 10th ed, pp 1525-1528</ref><ref name="crc1582">''CRC Handbook of Chemistry and Physics'' 44th ed. pp 2582-2584</ref> ''c<sub>p</sub>'' | |||
| 78.28 J/(mol K) at 90 °C<br>87.53 J/(mol K) at 110-220 °C | |||
|- | |||
| [[Heat capacity ratio]],<ref name="lange1525"/><ref name="crc1582"/><br> ''γ'' = ''c<sub>p</sub>''/''c<sub>v</sub>'' | |||
| 1.13 at 90 °C | |||
|- | |||
| [[van der Waals equation|van der Waals' constants]]<ref name="lange1522">''Lange's Handbook of Chemistry'' 10th ed, pp 1522-1524</ref> | |||
| a = 1217.9 L<sup>2</sup> kPa/mol<sup>2</sup><br> b = 0.08407 liter per mole | |||
|- | |||
|} | |||
== Spectral data == <!-- KEEP this header, it is linked to from the infobox on the main article page --> | |||
{| border="1" cellspacing="0" cellpadding="3" style="margin: 0 0 0 0.5em; background: #FFFFFF; border-collapse: collapse; border-color: #C0C090;" | |||
! {{chembox header}} | [[UV/VIS spectroscopy|UV-Vis]] | |||
|- | |||
| [[Lambda-max|λ<sub>max</sub>]] | |||
| ? [[Nanometre|nm]] | |||
|- | |||
| [[molar absorptivity|Extinction coefficient]], ε | |||
| ? | |||
|- | |||
! {{chembox header}} | [[Infrared|IR]] | |||
|- | |||
| Major absorption bands<ref name="aist">{{Cite web|url=http://riodb01.ibase.aist.go.jp/sdbs/cgi-bin/cre_index.cgi?lang=eng|title=Spectral Database for Organic Compounds|publisher=Advanced Industrial Science and Technology|format=Queriable database|accessdate=9 June 2007}}</ref> | |||
| | |||
{| | |||
|- | |||
| colspan="2" align="center" | (liquid film) | |||
|- | |||
! Wave number !! transmittance | |||
|- | |||
| 880 cm<sup>−1</sup> || 22% | |||
|- | |||
| 1048 cm<sup>−1</sup> || 6% | |||
|- | |||
| 1089 cm<sup>−1</sup> || 19% | |||
|- | |||
| 1275 cm<sup>−1</sup> || 52% | |||
|- | |||
| 1329 cm<sup>−1</sup> || 44% | |||
|- | |||
| 1384 cm<sup>−1</sup> || 32% | |||
|- | |||
| 1418 cm<sup>−1</sup> || 33% | |||
|- | |||
| 1650 cm<sup>−1</sup> || 26% | |||
|- | |||
| 1926 cm<sup>−1</sup> || 62% | |||
|- | |||
| 2899 cm<sup>−1</sup> || 12% | |||
|- | |||
| 2930 cm<sup>−1</sup> || 13% | |||
|- | |||
| 2977 cm<sup>−1</sup> || 4% | |||
|} | |||
|- | |||
! {{chembox header}} | [[NMR Spectroscopy|NMR]] | |||
|- | |||
| [[Proton NMR]] <!-- Link to image of spectrum --> | |||
| | |||
|- | |||
| [[Carbon-13 NMR]] <!-- Link to image of spectrum --> | |||
| http://riodb01.ibase.aist.go.jp/sdbs/cgi-bin/IMG.cgi?fname=CDS00245&imgdir=cdsW; | |||
|- | |||
| Other NMR data <!-- Insert special data e.g. <sup>19</sup>F chem. shifts, omit if not used --> | |||
| | |||
|- | |||
! {{chembox header}} | [[Mass Spectrometry|MS]] | |||
|- | |||
| Masses of <br>main fragments | |||
| <!-- Give list of major fragments --> | |||
|- | |||
|} | |||
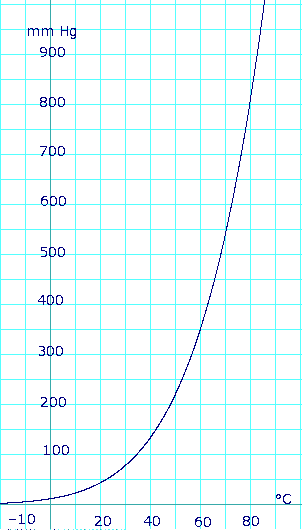
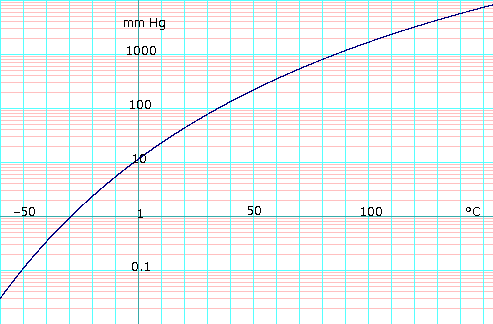
==Vapor pressure of liquid == | |||
{| | |||
|- | |||
| | |||
{| border="1" cellspacing="0" cellpadding="6" style="margin: 0 0 0 0.5em; background: white; border-collapse: collapse; border-color: #C0C090;" | |||
|- align=center | |||
| {{chembox header}} | '''P in mm Hg''' || 1 || 10 || 40 || 100 || 400 || 760 || 1520 || 3800 || 7600 || 15200 || 30400 || 45600 | |||
|- align=center | |||
| {{chembox header}} | '''P in Pa''' || 133.3 || 1333 || 5333 || 13332 || 53329 || 101325 || 202650 || 506625 || 1013250 || 2026500 || 4053000 || 6079501 | |||
|- align=center | |||
| {{chembox header}} | '''T in °C''' || –31.3 || –2.3 || 19.0 || 34.9 || 63.5 || 78.4 || 97.5 || 126.0 || 151.8 || 183.0 || 218.0 || 242.0 | |||
|- align=center | |||
| {{chembox header}} | '''T in K''' || 241.85 || 270.85 || 292.15 || 308.05 || 336.65 || 351.55 || 370.65 || 399.15 || 424.95 || 456.15 || 491.15 || 515.15 | |||
|} | |||
|- | |||
| | |||
{| | |||
| | |||
[[Image:EtOHvaporpressureL.png|thumb|302px|left|Ethanol vapor pressure vs. temperature. Uses formula: <math>\scriptstyle P_{mmHg} = 10^{8.04494 - \frac {1554.3} {222.65+T}}</math> from ''Lange's Handbook of Chemistry'' 10th ed.]] | |||
| | |||
[[Image:EtOHlogVaporPressureL.png|thumb|500px|left|Log<sub>10</sub> of ethanol vapor pressure vs. temperature. Uses formula: <math>\scriptstyle \log_{10} {P_{mmHg}} = 8.04494 - \frac {1554.3} {222.65+T}</math>]] | |||
|} | |||
|} | |||
{{Clear}} | |||
==Density of ethanol at various temperatures (''kg/l'' or ''g/cm<sup>3</sup>'')== | |||
Data obtained from ''Lange's Handbook of Chemistry'', 10th ed. | |||
{| border="1" cellspacing="0" cellpadding="6" style="margin: 0 0 0 0.5em; background: #FFFFFF; border-collapse: collapse; border-color: #C0C090;" | |||
|- {{chembox header}} bgcolor="#F8EABA" | |||
| 3 °C || 0.80374 || 16 °C || 0.79283 || 29 °C || 0.78182 | |||
|- | |||
| 4 °C || 0.80290 || 17 °C || 0.79198 || 30 °C || 0.78097 | |||
|- | |||
| 5 °C || 0.80207 || 18 °C || 0.79114 || 31 °C || 0.78012 | |||
|- | |||
| 6 °C || 0.80123 || 19 °C || 0.79029 || 32 °C || 0.77927 | |||
|- | |||
| 7 °C || 0.80039 || 20 °C || 0.78945 || 33 °C || 0.77841 | |||
|- | |||
| 8 °C || 0.79956 || 21 °C || 0.78860 || 34 °C || 0.77756 | |||
|- | |||
| 9 °C || 0.79872 || 22 °C || 0.78775 || 35 °C || 0.77671 | |||
|- | |||
| 10 °C || 0.79788 || 23 °C || 0.78691 || 36 °C || 0.77585 | |||
|- | |||
| 11 °C || 0.79704 || 24 °C || 0.78606 || 37 °C || 0.77500 | |||
|- | |||
| 12 °C || 0.79620 || 25 °C || 0.78522 || 38 °C || 0.77414 | |||
|- | |||
| || || 39 °C || 0.77329 || 40 °C || 0.77244 | |||
|} | |||
These data correlate as Density (g/cm<sup>3</sup>) = -8.461834E-4 T( °C) + 0.8063372 with an R<sup>2</sup> coefficient of determination of 0.99999. | |||
==Properties of aqueous ethanol solutions== | |||
Data obtained from ''Lange's Handbook of Chemistry'', 10th ed. The annotation, '''d a °C/b °C''', indicates density of solution at temperature '''a''' divided by density of pure water at temperature '''b'''. | |||
{| border="1" cellspacing="0" cellpadding="6" style="margin: 0 0 0 0.5em; background: #FFFFFF; border-collapse: collapse; border-color: #C0C090;" | |||
|- {{chembox header}} | |||
| '''% wt<br>ethanol''' || '''% vol<br>ethanol''' || '''grams<br>ethanol<br>per 100 cc<br>15.56 °C''' || '''d 10 °C/4 °C''' || '''d 20 °C/4 °C''' || '''d 25 °C/4 °C''' || '''d 30 °C/4 °C''' || '''d 20 °C/20 °C''' || '''d 25 °C/25 °C''' || '''freezing<br>temp.''' | |||
|- | |||
| 0.0 || 0.0 || 0.0 || 0.99973 || 0.99823 || 0.99708 || 0.99568 || 1.00000 || 1.00000 || 0 °C | |||
|- | |||
| 1.0 || || || 0.99785 || 0.99636 || 0.99520 || 0.99379 || 0.99813 || 0.99811 || | |||
|- | |||
| 2.0 || || || 0.99602 || 0.99453 || 0.99336 || 0.99194 || 0.99629 || 0.99627 || | |||
|- | |||
| 2.5 || 3.13 || || || 0.99363 || || || || || –1 °C | |||
|- | |||
| 3.0 || || || 0.99426 || 0.99275 || 0.99157 || 0.99014 || 0.99451 || 0.99447 || | |||
|- | |||
| 4.0 || 5.00 || 3.97 || 0.99258 || 0.99103 || 0.98984 || 0.98839 || 0.99279 || 0.99274 || | |||
|- | |||
| 4.8 || 6.00 || 4.76 || || 0.98971 || || || || || –2 °C | |||
|- | |||
| 5.0 || || || 0.99098 || 0.98938 || 0.98817 || 0.98670 || 0.99113 || 0.99106 || | |||
|- | |||
| 5.05 || 6.30 || 5.00 || || 0.98930 || || || || || | |||
|- | |||
| 6.0 || || || 0.98946 || 0.98780 || 0.98656 || 0.98507 || 0.98955 || 0.98945 || | |||
|- | |||
| 6.8 || 8.47 || || || 0.98658 || || || || || –3 °C | |||
|- | |||
| 7.0 || || || 0.98801 || 0.98627 || 0.98500 || 0.98347 || 0.98802 || 0.98788 || | |||
|- | |||
| 8.0 || || || 0.98660 || 0.98478 || 0.98346 || 0.98189 || 0.98653 || 0.98634 || | |||
|- | |||
| 9.0 || || || 0.98524 || 0.98331 || 0.98193 || 0.98031 || 0.98505 || 0.98481 || | |||
|- | |||
| 10.0 || 12.40 || 9.84 || 0.98393 || 0.98187 || 0.98043 || 0.97575 || 0.98361 || 0.98330 || | |||
|- | |||
| 11.0 || || || 0.98267 || 0.98047 || 0.97897 || 0.97723 || 0.98221 || 0.98184 || | |||
|- | |||
| 11.3 || 14.0 || 11.11 || || 0.98006 || || || || || –5 °C | |||
|- | |||
| 12.0 || || || 0.98145 || 0.97910 || 0.97753 || 0.97573 || 0.98084 || 0.98039 || | |||
|- | |||
| 13.0 || || || 0.98026 || 0.97775 || 0.97611 || 0.97424 || 0.97948 || 0.97897 || | |||
|- | |||
| 13.78 || 17.00 || 13.49 || || || || || || || –6.1 °C | |||
|- | |||
| 14.0 || || || 0.97911 || 0.97643 || 0.97472 || 0.97278 || 0.97816 || 0.97757 || | |||
|- | |||
| 15.0 || || || 0.97800 || 0.97514 || 0.97334 || 0.97133 || 0.97687 || 0.97619 || | |||
|- | |||
| 15.02 || 18.50 || 14.68 || || 0.97511 || || || || || | |||
|- | |||
| 16.0 || || || 0.97692 || 0.97387 || 0.97199 || 0.96990 || 0.97560 || 0.97484 || | |||
|- | |||
| 16.4 || 20.2 || || || 0.97336 || || || || || –7.5 °C | |||
|- | |||
| 17.0 || || || 0.97583 || 0.97259 || 0.97062 || 0.96844 || 0.97431 || 0.97346 || | |||
|- | |||
| 17.5 || 21.5 || || || 0.97194 || || || || || –8.7 °C | |||
|- | |||
| 18.0 || 22.10 || 17.54 || 0.97473 || 0.97129 || 0.96923 || 0.96697 || 0.97301 || 0.97207 || | |||
|- | |||
| 18.8 || 23.1 || || || 0.97024 || || || || || –9.4 °C | |||
|- | |||
| 19.0 || || || 0.97363 || 0.96997 || 0.96782 || 0.96547 || 0.97169 || 0.97065 || | |||
|- | |||
| 20.0 || || || 0.97252 || 0.96864 || 0.96639 || 0.96395 || 0.97036 || 0.96922 || | |||
|- | |||
| 20.01 || 24.50 || 19.44 || || 0.96863 || || || || || | |||
|- | |||
| 20.3 || 24.8 || || || 0.96823 || || || || || –10.6 °C | |||
|- | |||
| 21.0 || || || 0.97139 || 0.96729 || 0.96495 || 0.96242 || 0.96901 || 0.96778 || | |||
|- | |||
| 22.0 || || || 0.97024 || 0.96592 || 0.96348 || 0.96087 || 0.96763 || 0.96630 || | |||
|- | |||
| 22.11 || 27.00 || 21.43 || || 0.96578 || || || || || –12.2 °C | |||
|- | |||
| 23.0 || || || 0.96907 || 0.96453 || 0.96199 || 0.95929 || 0.96624 || 0.96481 || | |||
|- | |||
| 24.0 || || || 0.96787 || 0.96312 || 0.96048 || 0.95769 || 0.96483 || 0.96329 || | |||
|- | |||
| 24.2 || 29.5 || || || 0.96283 || || || || || –14.0 °C | |||
|- | |||
| 25.0 || 30.40 || 24.12 || 0.96665 || 0.96168 || 0.95895 || 0.95607 || 0.96339 || 0.96176 || | |||
|- | |||
| 26.0 || || || 0.96539 || 0.96020 || 0.95738 || 0.95422 || 0.96190 || 0.96018 || | |||
|- | |||
| 26.7 || 32.4 || || || 0.95914 || || || || || –16.0 °C | |||
|- | |||
| 27.0 || || || 0.96406 || 0.95867 || 0.95576 || 0.95272 || 0.96037 || 0.95856 || | |||
|- | |||
| 28.0 || 33.90 || 26.90 || 0.96268 || 0.95710 || 0.95410 || 0.95098 || 0.95880 || 0.95689 || | |||
|- | |||
| 29.0 || || || 0.96125 || 0.95548 || 0.95241 || 0.94922 || 0.95717 || 0.95520 || | |||
|- | |||
| 29.9 || 36.1 || || || 0.95400 || || || || || –18.9 °C | |||
|- | |||
| 30.0 || 36.20 || 28.73 || 0.95977 || 0.95382 || 0.95067 || 0.94741 || 0.95551 || 0.95345 || | |||
|- | |||
| 31.0 || || || 0.95823 || 0.95212 || 0.94890 || 0.94557 || 0.95381 || 0.95168 || | |||
|- | |||
| 32.0 || || || 0.95665 || 0.95038 || 0.94709 || 0.94370 || 0.95207 || 0.94986 || | |||
|- | |||
| 33.0 || || || 0.95502 || 0.94860 || 0.94525 || 0.94180 || 0.95028 || 0.94802 || | |||
|- | |||
| 33.8 || 40.5 || || || 0.94715 || || || || || –23.6 °C | |||
|- | |||
| 34.0 || || || 0.95334 || 0.94679 || 0.94337 || 0.93986 || 0.94847 || 0.94613 || | |||
|- | |||
| 35.0 || || || 0.95162 || 0.94494 || 0.94146 || 0.93790 || 0.94662 || 0.94422 || | |||
|- | |||
| 35.04 || 41.90 || 33.25 || || 0.94486 || || || || || | |||
|- | |||
| 36.0 || || || 0.94986 || 0.94306 || 0.93952 || 0.93591 || 0.94473 || 0.94227 || | |||
|- | |||
| 37.0 || || || 0.94805 || 0.94114 || 0.93756 || 0.93390 || 0.94281 || 0.94031 || | |||
|- | |||
| 38.0 || || || 0.94620 || 0.93919 || 0.93556 || 0.93186 || 0.94086 || 0.93830 || | |||
|- | |||
| 39.0 || 46.3 || || 0.94431 || 0.93720 || 0.93353 || 0.92979 || 0.93886 || 0.93626 || –28.7 °C | |||
|- | |||
| 40.0 || || || 0.94238 || 0.93518 || 0.93148 || 0.92770 || 0.93684 || 0.93421 || | |||
|- | |||
| 40.04 || 47.40 || 37.61 || || 0.93510 || || || || || | |||
|- | |||
| 41.0 || || || 0.94042 || 0.93314 || 0.92940 || 0.92558 || 0.93479 || 0.93212 || | |||
|- | |||
| 42.0 || || || 0.93842 || 0.93107 || 0.92729 || 0.92344 || 0.93272 || 0.93001 || | |||
|- | |||
| 43.0 || || || 0.93639 || 0.92897 || 0.92516 || 0.92128 || 0.93062 || 0.92787 || | |||
|- | |||
| 44.0 || || || 0.93433 || 0.92685 || 0.92301 || 0.91910 || 0.92849 || 0.92571 || | |||
|- | |||
| 45.0 || || || 0.93226 || 0.92472 || 0.92085 || 0.91692 || 0.92636 || 0.92355 || | |||
|- | |||
| 45.31 || 53.00 || 42.07 || || 0.92406 || || || || || | |||
|- | |||
| 46.0 || || || 0.93017 || 0.92257 || 0.91868 || 0.91472 || 0.92421 || 0.92137 || | |||
|- | |||
| 46.3 || 53.8 || || || 0.92193 || || || || || –33.9 °C | |||
|- | |||
| 47.0 || || || 0.92806 || 0.92041 || 0.91649 || 0.91250 || 0.92204 || 0.91917 || | |||
|- | |||
| 48.0 || || || 0.92593 || 0.91823 || 0.91429 || 0.91028 || 0.91986 || 0.91697 || | |||
|- | |||
| 49.0 || || || 0.92379 || 0.91604 || 0.91208 || 0.90805 || 0.91766 || 0.91475 || | |||
|- | |||
| 50.0 || || || 0.92162 || 0.91384 || 0.90985 || 0.90580 || 0.91546 || 0.91251 || | |||
|- | |||
| 50.16 || 58.0 || 46.04 || || 0.91349 || || || || || | |||
|- | |||
| 51.0 || || || 0.91943 || 0.91160 || 0.90760 || 0.90353 || 0.91322 || 0.91026 || | |||
|- | |||
| 52.0 || || || 0.91723 || 0.90936 || 0.90524 || 0.90125 || 0.91097 || 0.90799 || | |||
|- | |||
| 53.0 || || || 0.91502 || 0.90711 || 0.90307 || 0.89896 || 0.90872 || 0.90571 || | |||
|- | |||
| 54.0 || || || 0.91279 || 0.90485 || 0.90079 || 0.89667 || 0.90645 || 0.90343 || | |||
|- | |||
| 55.0 || || || 0.91055 || 0.90258 || 0.89850 || 0.89437 || 0.90418 || 0.90113 || | |||
|- | |||
| 55.16 || 63.0 || 50.00 || || 0.90220 || || || || || | |||
|- | |||
| 56.0 || || || 0.90831 || 0.90031 || 0.89621 || 0.89206 || 0.90191 || 0.89833 || | |||
|- | |||
| 56.1 || 63.6 || || || 0.90008 || || || || || –41.0 °C | |||
|- | |||
| 57.0 || || || 0.90607 || 0.89803 || 0.89392 || 0.88975 || 0.89962 || 0.89654 || | |||
|- | |||
| 58.0 || || || 0.90381 || 0.89574 || 0.89162 || 0.88744 || 0.89733 || 0.89423 || | |||
|- | |||
| 59.0 || || || 0.90154 || 0.89344 || 0.88931 || 0.88512 || 0.89502 || 0.89191 || | |||
|- | |||
| 60.0 || || || 0.89927 || 0.89113 || 0.88699 || 0.88278 || 0.89271 || 0.88959 || | |||
|- | |||
| 60.33 || 68.0 || 53.98 || || 0.89038 || || || || || | |||
|- | |||
| 61.0 || || || 0.89898 || 0.88882 || 0.88466 || 0.88044 || 0.89040 || 0.88725 || | |||
|- | |||
| 62.0 || || || 0.89468 || 0.88650 || 0.88233 || 0.87809 || 0.88807 || 0.88491 || | |||
|- | |||
| 63.0 || || || 0.89237 || 0.88417 || 0.87998 || 0.87574 || 0.88574 || 0.88256 || | |||
|- | |||
| 64.0 || || || 0.89006 || 0.88183 || 0.87763 || 0.87337 || 0.88339 || 0.88020 || | |||
|- | |||
| 65.0 || || || 0.88774 || 0.87948 || 0.87527 || 0.87100 || 0.88104 || 0.87783 || | |||
|- | |||
| 66.0 || || || 0.88541 || 0.87713 || 0.87291 || 0.86863 || 0.87869 || 0.87547 || | |||
|- | |||
| 67.0 || || || 0.88308 || 0.87477 || 0.87054 || 0.86625 || 0.87632 || 0.87309 || | |||
|- | |||
| 68.0 || || || 0.88071 || 0.87241 || 0.86817 || 0.86387 || 0.87396 || 0.87071 || | |||
|- | |||
| 69.0 || || || 0.87839 || 0.87004 || 0.86579 || 0.86148 || 0.87158 || 0.86833 || | |||
|- | |||
| 70.0 || || || 0.87602 || 0.86766 || 0.86340 || 0.85908 || 0.86920 || 0.86593 || | |||
|- | |||
| 71.0 || || || 0.87365 || 0.86527 || 0.86100 || 0.85667 || 0.86680 || 0.86352 || | |||
|- | |||
| 71.9 || 78.3 || || || 0.86311 || || || || || –51.3 °C | |||
|- | |||
| 72.0 || || || 0.87127 || 0.86287 || 0.85859 || 0.85426 || 0.86440 || 0.86110 || | |||
|- | |||
| 73.0 || || || 0.86888 || 0.86047 || 0.85618 || 0.85184 || 0.86200 || 0.85869 || | |||
|- | |||
| 74.0 || || || 0.86648 || 0.85806 || 0.85376 || 0.84941 || 0.85958 || 0.85626 || | |||
|- | |||
| 75.0 || || || 0.86408 || 0.85564 || 0.85135 || 0.84698 || 0.85716 || 0.85383 || | |||
|- | |||
| 76.0 || || || 0.86168 || 0.85322 || 0.84891 || 0.84455 || 0.85473 || 0.85140 || | |||
|- | |||
| 77.0 || || || 0.85927 || 0.85079 || 0.84647 || 0.84211 || 0.85230 || 0.84895 || | |||
|- | |||
| 78.0 || || || 0.85685 || 0.84835 || 0.84403 || 0.83966 || 0.84985 || 0.84650 || | |||
|- | |||
| 79.0 || || || 0.85422 || 0.84590 || 0.84158 || 0.83720 || 0.84740 || 0.84404 || | |||
|- | |||
| 80.0 || || || 0.85197 || 0.84344 || 0.83911 || 0.83473 || 0.84494 || 0.84157 || | |||
|- | |||
| 81.0 || || || 0.84950 || 0.84096 || 0.83664 || 0.83224 || 0.84245 || 0.83909 || | |||
|- | |||
| 82.0 || || || 0.84702 || 0.83848 || 0.83415 || 0.82974 || 0.83997 || 0.83659 || | |||
|- | |||
| 83.0 || || || 0.84453 || 0.83599 || 0.83164 || 0.82724 || 0.83747 || 0.83408 || | |||
|- | |||
| 84.0 || || || 0.84203 || 0.83348 || 0.82913 || 0.82473 || 0.83496 || 0.83156 || | |||
|- | |||
| 85.0 || || || 0.83951 || 0.83095 || 0.82660 || 0.82220 || 0.83242 || 0.82902 || | |||
|- | |||
| 86.0 || || || 0.83697 || 0.82840 || 0.82405 || 0.81965 || 0.82987 || 0.82646 || | |||
|- | |||
| 87.0 || || || 0.83441 || 0.82554 || 0.82148 || 0.81708 || 0.82729 || 0.82389 || | |||
|- | |||
| 88.0 || || || 0.83181 || 0.82323 || 0.81888 || 0.81448 || 0.82469 || 0.82128 || | |||
|- | |||
| 89.0 || || || 0.82919 || 0.82062 || 0.81626 || 0.81186 || 0.82207 || 0.81865 || | |||
|- | |||
| 90.0 || || || 0.82654 || 0.81797 || 0.81362 || 0.80922 || 0.81942 || 0.81600 || | |||
|- | |||
| 91.00 || 94.00 || 74.62 || 0.82386 || 0.81529 || 0.81094 || 0.80655 || 0.81674 || 0.81331 || | |||
|- | |||
| 92.0 || || || 0.82114 || 0.81257 || 0.80823 || 0.80384 || 0.81401 || 0.81060 || | |||
|- | |||
| 93.0 || || || 0.81839 || 0.80983 || 0.80549 || 0.80111 || 0.81127 || 0.80785 || | |||
|- | |||
| 94.0 || || || 0.81561 || 0.80705 || 0.80272 || 0.79835 || 0.80848 || 0.80507 || | |||
|- | |||
| 95.0 || || || 0.81278 || 0.80424 || 0.79991 || 0.79555 || 0.80567 || 0.80225 || | |||
|- | |||
| 96.0 || || || 0.80991 || 0.80138 || 0.79706 || 0.79271 || 0.80280 || 0.79939 || | |||
|- | |||
| 97.0 || || || 0.80698 || 0.79846 || 0.79415 || 0.78981 || 0.79988 || 0.79648 || | |||
|- | |||
| 98.0 || || || 0.80399 || 0.79547 || 0.79117 || 0.78684 || 0.79688 || 0.79349 || | |||
|- | |||
| 99.0 || || || 0.80094 || 0.79243 || 0.78814 || 0.78382 || 0.79383 || 0.79045 || | |||
|- | |||
| 100.0 || 100.0 || 79.39 || 0.79784 || 0.78934 || 0.78506 || 0.78075 || 0.79074 || 0.78736 || −114.3 °C | |||
|- {{chembox header}} | |||
| '''% wt<br>ethanol''' || '''% vol<br>ethanol''' || '''grams<br>ethanol<br>per 100 cc<br>15.56 °C''' || '''d 10 °C/4 °C''' || '''d 20 °C/4 °C''' || '''d 25 °C/4 °C''' || '''d 30 °C/4 °C''' || '''d 20 °C/20 °C''' || '''d 25 °C/25 °C''' || '''freezing<br>temp.''' | |||
|} | |||
==Boiling points of aqueous solutions==<!-- This section is linked from [[Azeotrope]] --> | |||
Data obtained from ''CRC Handbook of Chemistry'' 44th ed., p2391 | |||
{| border="1" cellspacing="0" cellpadding="6" style="margin: 0 0 0 0.5em; background: white; border-collapse: collapse; border-color: #C0C090;" | |||
|- {{chembox header}} | |||
| rowspan="2" | '''BP °C''' | |||
| colspan="2" | '''Weight % ethanol''' | |||
| rowspan="2" | | |||
| rowspan="2" | '''BP °C''' | |||
| colspan="2" | '''Weight % ethanol''' | |||
|- {{chembox header}} | |||
| '''liquid''' || '''vapor''' || '''liquid''' || '''vapor''' | |||
|- | |||
| 78.1 || 95.5<sup>‡</sup> || 95.5<sup>‡</sup> | |||
| rowspan="29" | | |||
| || || | |||
|- | |||
| 78.2 || 91 || 92 || 86.5 || 18 || 71 | |||
|- | |||
| 78.4 || 85 || 89 || 87.0 || 17 || 70 | |||
|- | |||
| 78.6 || 82 || 88 || 87.5 || 16 || 69 | |||
|- | |||
| 78.8 || 80 || 87 || 88.0 || 15 || 68 | |||
|- | |||
| 79.0 || 78 || 86 || 88.5 || 13 || 67 | |||
|- | |||
| 79.2 || 76 || 85 || 89.0 || 12 || 65 | |||
|- | |||
| 79.4 || 74 || 85 || 89.5 || 11 || 63 | |||
|- | |||
| 79.6 || 72 || 84 || 90.0 || 10 || 61 | |||
|- | |||
| 79.8 || 69 || 84 || 90.5 || 10 || 59 | |||
|- | |||
| 80.0 || 67 || 83 || 91.0 || 9 || 57 | |||
|- | |||
| 80.2 || 64 || 83 || 91.5 || 8 || 55 | |||
|- | |||
| 80.4 || 62 || 82 || 92.0 || 8 || 53 | |||
|- | |||
| 80.6 || 59 || 82 || 92.5 || 7 || 51 | |||
|- | |||
| 80.8 || 56 || 81 || 93.0 || 6 || 49 | |||
|- | |||
| 81.0 || 53 || 81 || 93.5 || 6 || 46 | |||
|- | |||
| 81.2 || 50 || 80 || 94.0 || 5 || 44 | |||
|- | |||
| 81.4 || 47 || 80 || 94.5 || 5 || 42 | |||
|- | |||
| 81.6 || 45 || 80 || 95.0 || 4 || 39 | |||
|- | |||
| 81.8 || 43 || 79 || 95.5 || 4 || 36 | |||
|- | |||
| 82.0 || 41 || 79 || 96.0 || 3 || 33 | |||
|- | |||
| 82.5 || 36 || 78 || 96.5 || 3 || 30 | |||
|- | |||
| 83.0 || 33 || 78 || 97.0 || 2 || 27 | |||
|- | |||
| 83.5 || 30 || 77 || 97.5 || 2 || 23 | |||
|- | |||
| 84.0 || 27 || 77 || 98.0 || 1 || 19 | |||
|- | |||
| 84.5 || 25 || 75 || 98.5 || 1 || 15 | |||
|- | |||
| 85.0 || 23 || 74 || 99.0 || < 1 || 10 | |||
|- | |||
| 85.5 || 21 || 73 || 99.5 || < 1 || 5 | |||
|- | |||
| 86.0 || 20 || 72 || 100.0 || 0 || 0 | |||
|} | |||
<sup>‡</sup>[[azeotrope|Azeotropic mixture]] | |||
==References== | |||
<references/> | |||
*{{nist}} | |||
{{DEFAULTSORT:Ethanol (Data Page)}} | |||
[[Category:Chemical data pages]] | |||
Revision as of 20:06, 11 January 2014
30 year-old Entertainer or Range Artist Wesley from Drumheller, really loves vehicle, property developers properties for sale in singapore singapore and horse racing. Finds inspiration by traveling to Works of Antoni Gaudí. Template:Chembox
This page provides supplementary chemical data on ethanol. Except where noted otherwise, data relate to standard ambient temperature and pressure.
Material Safety Data Sheet
The handling of this chemical may incur notable safety precautions. It is highly recommend that you seek the Material Safety Datasheet (MSDS) for this chemical from a reliable source and follow its directions.
Structure and properties
| Structure and properties | |||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Index of refraction, n25 | 1.361 | ||||||||||||||||||||||||||||||||||||||
| Dielectric constant, εr | 24.3 ε0 at 25 °C | ||||||||||||||||||||||||||||||||||||||
| Bond strength | ? | ||||||||||||||||||||||||||||||||||||||
| Bond length | ? | ||||||||||||||||||||||||||||||||||||||
| Bond angle | ? | ||||||||||||||||||||||||||||||||||||||
| Magnetic susceptibility[1] | 5.8·10−7 (cgs units, volume) | ||||||||||||||||||||||||||||||||||||||
| Surface tension | 22.39 dyn/cm at 25 °C | ||||||||||||||||||||||||||||||||||||||
| Viscosity[2] |
|
Thermodynamic properties
| Phase behavior | |
|---|---|
| Triple point | 150 K (−123 °C), 0.00043 Pa |
| Critical point | 514 K (241 °C), 63 bar |
| Std enthalpy change of fusion, ΔfusH |
+4.9 kJ/mol |
| Std entropy change of fusion, ΔfusS |
+31 J/(mol·K) |
| Std enthalpy change of vaporization, ΔvapH |
+38.56 kJ/mol |
| Std entropy change of vaporization, ΔvapS |
109.67 J/(mol·K) |
| Molal freezing point constant | –1.99 °C kg/mol |
| Molal boiling point constant | 1.19 °C kg/mol |
| Solid properties | |
| Std enthalpy change of formation, ΔfH |
–277.7 kJ/mol |
| Standard molar entropy, S |
160.7 J/(mol K)[3] |
| Heat capacity, cp | 111.46 J/(mol K) [3] |
| Liquid properties | |
| Std enthalpy change of formation, ΔfH |
−277.38 kJ/mol |
| Standard molar entropy, S |
159.9 J/(mol K) |
| Enthalpy of combustion, ΔcH |
–1370.7 kJ/mol |
| Heat capacity, cp | 112.4 J/(mol K) |
| Gas properties | |
| Std enthalpy change of formation, ΔfH |
−235.3 kJ/mol |
| Standard molar entropy, S |
283 J/(mol K) |
| Heat capacity,[4][5] cp | 78.28 J/(mol K) at 90 °C 87.53 J/(mol K) at 110-220 °C |
| Heat capacity ratio,[4][5] γ = cp/cv |
1.13 at 90 °C |
| van der Waals' constants[6] | a = 1217.9 L2 kPa/mol2 b = 0.08407 liter per mole |
Spectral data
| UV-Vis | |||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| λmax | ? nm | ||||||||||||||||||||||||||||
| Extinction coefficient, ε | ? | ||||||||||||||||||||||||||||
| IR | |||||||||||||||||||||||||||||
| Major absorption bands[7] |
| ||||||||||||||||||||||||||||
| NMR | |||||||||||||||||||||||||||||
| Proton NMR | |||||||||||||||||||||||||||||
| Carbon-13 NMR | http://riodb01.ibase.aist.go.jp/sdbs/cgi-bin/IMG.cgi?fname=CDS00245&imgdir=cdsW; | ||||||||||||||||||||||||||||
| Other NMR data | |||||||||||||||||||||||||||||
| MS | |||||||||||||||||||||||||||||
| Masses of main fragments |
|||||||||||||||||||||||||||||
Vapor pressure of liquid
50 year old Petroleum Engineer Kull from Dawson Creek, spends time with interests such as house brewing, property developers in singapore condo launch and camping. Discovers the beauty in planing a trip to places around the entire world, recently only coming back from .
Density of ethanol at various temperatures (kg/l or g/cm3)
Data obtained from Lange's Handbook of Chemistry, 10th ed.
| 3 °C | 0.80374 | 16 °C | 0.79283 | 29 °C | 0.78182 |
| 4 °C | 0.80290 | 17 °C | 0.79198 | 30 °C | 0.78097 |
| 5 °C | 0.80207 | 18 °C | 0.79114 | 31 °C | 0.78012 |
| 6 °C | 0.80123 | 19 °C | 0.79029 | 32 °C | 0.77927 |
| 7 °C | 0.80039 | 20 °C | 0.78945 | 33 °C | 0.77841 |
| 8 °C | 0.79956 | 21 °C | 0.78860 | 34 °C | 0.77756 |
| 9 °C | 0.79872 | 22 °C | 0.78775 | 35 °C | 0.77671 |
| 10 °C | 0.79788 | 23 °C | 0.78691 | 36 °C | 0.77585 |
| 11 °C | 0.79704 | 24 °C | 0.78606 | 37 °C | 0.77500 |
| 12 °C | 0.79620 | 25 °C | 0.78522 | 38 °C | 0.77414 |
| 39 °C | 0.77329 | 40 °C | 0.77244 |
These data correlate as Density (g/cm3) = -8.461834E-4 T( °C) + 0.8063372 with an R2 coefficient of determination of 0.99999.
Properties of aqueous ethanol solutions
Data obtained from Lange's Handbook of Chemistry, 10th ed. The annotation, d a °C/b °C, indicates density of solution at temperature a divided by density of pure water at temperature b.
| % wt ethanol |
% vol ethanol |
grams ethanol per 100 cc 15.56 °C |
d 10 °C/4 °C | d 20 °C/4 °C | d 25 °C/4 °C | d 30 °C/4 °C | d 20 °C/20 °C | d 25 °C/25 °C | freezing temp. |
| 0.0 | 0.0 | 0.0 | 0.99973 | 0.99823 | 0.99708 | 0.99568 | 1.00000 | 1.00000 | 0 °C |
| 1.0 | 0.99785 | 0.99636 | 0.99520 | 0.99379 | 0.99813 | 0.99811 | |||
| 2.0 | 0.99602 | 0.99453 | 0.99336 | 0.99194 | 0.99629 | 0.99627 | |||
| 2.5 | 3.13 | 0.99363 | –1 °C | ||||||
| 3.0 | 0.99426 | 0.99275 | 0.99157 | 0.99014 | 0.99451 | 0.99447 | |||
| 4.0 | 5.00 | 3.97 | 0.99258 | 0.99103 | 0.98984 | 0.98839 | 0.99279 | 0.99274 | |
| 4.8 | 6.00 | 4.76 | 0.98971 | –2 °C | |||||
| 5.0 | 0.99098 | 0.98938 | 0.98817 | 0.98670 | 0.99113 | 0.99106 | |||
| 5.05 | 6.30 | 5.00 | 0.98930 | ||||||
| 6.0 | 0.98946 | 0.98780 | 0.98656 | 0.98507 | 0.98955 | 0.98945 | |||
| 6.8 | 8.47 | 0.98658 | –3 °C | ||||||
| 7.0 | 0.98801 | 0.98627 | 0.98500 | 0.98347 | 0.98802 | 0.98788 | |||
| 8.0 | 0.98660 | 0.98478 | 0.98346 | 0.98189 | 0.98653 | 0.98634 | |||
| 9.0 | 0.98524 | 0.98331 | 0.98193 | 0.98031 | 0.98505 | 0.98481 | |||
| 10.0 | 12.40 | 9.84 | 0.98393 | 0.98187 | 0.98043 | 0.97575 | 0.98361 | 0.98330 | |
| 11.0 | 0.98267 | 0.98047 | 0.97897 | 0.97723 | 0.98221 | 0.98184 | |||
| 11.3 | 14.0 | 11.11 | 0.98006 | –5 °C | |||||
| 12.0 | 0.98145 | 0.97910 | 0.97753 | 0.97573 | 0.98084 | 0.98039 | |||
| 13.0 | 0.98026 | 0.97775 | 0.97611 | 0.97424 | 0.97948 | 0.97897 | |||
| 13.78 | 17.00 | 13.49 | –6.1 °C | ||||||
| 14.0 | 0.97911 | 0.97643 | 0.97472 | 0.97278 | 0.97816 | 0.97757 | |||
| 15.0 | 0.97800 | 0.97514 | 0.97334 | 0.97133 | 0.97687 | 0.97619 | |||
| 15.02 | 18.50 | 14.68 | 0.97511 | ||||||
| 16.0 | 0.97692 | 0.97387 | 0.97199 | 0.96990 | 0.97560 | 0.97484 | |||
| 16.4 | 20.2 | 0.97336 | –7.5 °C | ||||||
| 17.0 | 0.97583 | 0.97259 | 0.97062 | 0.96844 | 0.97431 | 0.97346 | |||
| 17.5 | 21.5 | 0.97194 | –8.7 °C | ||||||
| 18.0 | 22.10 | 17.54 | 0.97473 | 0.97129 | 0.96923 | 0.96697 | 0.97301 | 0.97207 | |
| 18.8 | 23.1 | 0.97024 | –9.4 °C | ||||||
| 19.0 | 0.97363 | 0.96997 | 0.96782 | 0.96547 | 0.97169 | 0.97065 | |||
| 20.0 | 0.97252 | 0.96864 | 0.96639 | 0.96395 | 0.97036 | 0.96922 | |||
| 20.01 | 24.50 | 19.44 | 0.96863 | ||||||
| 20.3 | 24.8 | 0.96823 | –10.6 °C | ||||||
| 21.0 | 0.97139 | 0.96729 | 0.96495 | 0.96242 | 0.96901 | 0.96778 | |||
| 22.0 | 0.97024 | 0.96592 | 0.96348 | 0.96087 | 0.96763 | 0.96630 | |||
| 22.11 | 27.00 | 21.43 | 0.96578 | –12.2 °C | |||||
| 23.0 | 0.96907 | 0.96453 | 0.96199 | 0.95929 | 0.96624 | 0.96481 | |||
| 24.0 | 0.96787 | 0.96312 | 0.96048 | 0.95769 | 0.96483 | 0.96329 | |||
| 24.2 | 29.5 | 0.96283 | –14.0 °C | ||||||
| 25.0 | 30.40 | 24.12 | 0.96665 | 0.96168 | 0.95895 | 0.95607 | 0.96339 | 0.96176 | |
| 26.0 | 0.96539 | 0.96020 | 0.95738 | 0.95422 | 0.96190 | 0.96018 | |||
| 26.7 | 32.4 | 0.95914 | –16.0 °C | ||||||
| 27.0 | 0.96406 | 0.95867 | 0.95576 | 0.95272 | 0.96037 | 0.95856 | |||
| 28.0 | 33.90 | 26.90 | 0.96268 | 0.95710 | 0.95410 | 0.95098 | 0.95880 | 0.95689 | |
| 29.0 | 0.96125 | 0.95548 | 0.95241 | 0.94922 | 0.95717 | 0.95520 | |||
| 29.9 | 36.1 | 0.95400 | –18.9 °C | ||||||
| 30.0 | 36.20 | 28.73 | 0.95977 | 0.95382 | 0.95067 | 0.94741 | 0.95551 | 0.95345 | |
| 31.0 | 0.95823 | 0.95212 | 0.94890 | 0.94557 | 0.95381 | 0.95168 | |||
| 32.0 | 0.95665 | 0.95038 | 0.94709 | 0.94370 | 0.95207 | 0.94986 | |||
| 33.0 | 0.95502 | 0.94860 | 0.94525 | 0.94180 | 0.95028 | 0.94802 | |||
| 33.8 | 40.5 | 0.94715 | –23.6 °C | ||||||
| 34.0 | 0.95334 | 0.94679 | 0.94337 | 0.93986 | 0.94847 | 0.94613 | |||
| 35.0 | 0.95162 | 0.94494 | 0.94146 | 0.93790 | 0.94662 | 0.94422 | |||
| 35.04 | 41.90 | 33.25 | 0.94486 | ||||||
| 36.0 | 0.94986 | 0.94306 | 0.93952 | 0.93591 | 0.94473 | 0.94227 | |||
| 37.0 | 0.94805 | 0.94114 | 0.93756 | 0.93390 | 0.94281 | 0.94031 | |||
| 38.0 | 0.94620 | 0.93919 | 0.93556 | 0.93186 | 0.94086 | 0.93830 | |||
| 39.0 | 46.3 | 0.94431 | 0.93720 | 0.93353 | 0.92979 | 0.93886 | 0.93626 | –28.7 °C | |
| 40.0 | 0.94238 | 0.93518 | 0.93148 | 0.92770 | 0.93684 | 0.93421 | |||
| 40.04 | 47.40 | 37.61 | 0.93510 | ||||||
| 41.0 | 0.94042 | 0.93314 | 0.92940 | 0.92558 | 0.93479 | 0.93212 | |||
| 42.0 | 0.93842 | 0.93107 | 0.92729 | 0.92344 | 0.93272 | 0.93001 | |||
| 43.0 | 0.93639 | 0.92897 | 0.92516 | 0.92128 | 0.93062 | 0.92787 | |||
| 44.0 | 0.93433 | 0.92685 | 0.92301 | 0.91910 | 0.92849 | 0.92571 | |||
| 45.0 | 0.93226 | 0.92472 | 0.92085 | 0.91692 | 0.92636 | 0.92355 | |||
| 45.31 | 53.00 | 42.07 | 0.92406 | ||||||
| 46.0 | 0.93017 | 0.92257 | 0.91868 | 0.91472 | 0.92421 | 0.92137 | |||
| 46.3 | 53.8 | 0.92193 | –33.9 °C | ||||||
| 47.0 | 0.92806 | 0.92041 | 0.91649 | 0.91250 | 0.92204 | 0.91917 | |||
| 48.0 | 0.92593 | 0.91823 | 0.91429 | 0.91028 | 0.91986 | 0.91697 | |||
| 49.0 | 0.92379 | 0.91604 | 0.91208 | 0.90805 | 0.91766 | 0.91475 | |||
| 50.0 | 0.92162 | 0.91384 | 0.90985 | 0.90580 | 0.91546 | 0.91251 | |||
| 50.16 | 58.0 | 46.04 | 0.91349 | ||||||
| 51.0 | 0.91943 | 0.91160 | 0.90760 | 0.90353 | 0.91322 | 0.91026 | |||
| 52.0 | 0.91723 | 0.90936 | 0.90524 | 0.90125 | 0.91097 | 0.90799 | |||
| 53.0 | 0.91502 | 0.90711 | 0.90307 | 0.89896 | 0.90872 | 0.90571 | |||
| 54.0 | 0.91279 | 0.90485 | 0.90079 | 0.89667 | 0.90645 | 0.90343 | |||
| 55.0 | 0.91055 | 0.90258 | 0.89850 | 0.89437 | 0.90418 | 0.90113 | |||
| 55.16 | 63.0 | 50.00 | 0.90220 | ||||||
| 56.0 | 0.90831 | 0.90031 | 0.89621 | 0.89206 | 0.90191 | 0.89833 | |||
| 56.1 | 63.6 | 0.90008 | –41.0 °C | ||||||
| 57.0 | 0.90607 | 0.89803 | 0.89392 | 0.88975 | 0.89962 | 0.89654 | |||
| 58.0 | 0.90381 | 0.89574 | 0.89162 | 0.88744 | 0.89733 | 0.89423 | |||
| 59.0 | 0.90154 | 0.89344 | 0.88931 | 0.88512 | 0.89502 | 0.89191 | |||
| 60.0 | 0.89927 | 0.89113 | 0.88699 | 0.88278 | 0.89271 | 0.88959 | |||
| 60.33 | 68.0 | 53.98 | 0.89038 | ||||||
| 61.0 | 0.89898 | 0.88882 | 0.88466 | 0.88044 | 0.89040 | 0.88725 | |||
| 62.0 | 0.89468 | 0.88650 | 0.88233 | 0.87809 | 0.88807 | 0.88491 | |||
| 63.0 | 0.89237 | 0.88417 | 0.87998 | 0.87574 | 0.88574 | 0.88256 | |||
| 64.0 | 0.89006 | 0.88183 | 0.87763 | 0.87337 | 0.88339 | 0.88020 | |||
| 65.0 | 0.88774 | 0.87948 | 0.87527 | 0.87100 | 0.88104 | 0.87783 | |||
| 66.0 | 0.88541 | 0.87713 | 0.87291 | 0.86863 | 0.87869 | 0.87547 | |||
| 67.0 | 0.88308 | 0.87477 | 0.87054 | 0.86625 | 0.87632 | 0.87309 | |||
| 68.0 | 0.88071 | 0.87241 | 0.86817 | 0.86387 | 0.87396 | 0.87071 | |||
| 69.0 | 0.87839 | 0.87004 | 0.86579 | 0.86148 | 0.87158 | 0.86833 | |||
| 70.0 | 0.87602 | 0.86766 | 0.86340 | 0.85908 | 0.86920 | 0.86593 | |||
| 71.0 | 0.87365 | 0.86527 | 0.86100 | 0.85667 | 0.86680 | 0.86352 | |||
| 71.9 | 78.3 | 0.86311 | –51.3 °C | ||||||
| 72.0 | 0.87127 | 0.86287 | 0.85859 | 0.85426 | 0.86440 | 0.86110 | |||
| 73.0 | 0.86888 | 0.86047 | 0.85618 | 0.85184 | 0.86200 | 0.85869 | |||
| 74.0 | 0.86648 | 0.85806 | 0.85376 | 0.84941 | 0.85958 | 0.85626 | |||
| 75.0 | 0.86408 | 0.85564 | 0.85135 | 0.84698 | 0.85716 | 0.85383 | |||
| 76.0 | 0.86168 | 0.85322 | 0.84891 | 0.84455 | 0.85473 | 0.85140 | |||
| 77.0 | 0.85927 | 0.85079 | 0.84647 | 0.84211 | 0.85230 | 0.84895 | |||
| 78.0 | 0.85685 | 0.84835 | 0.84403 | 0.83966 | 0.84985 | 0.84650 | |||
| 79.0 | 0.85422 | 0.84590 | 0.84158 | 0.83720 | 0.84740 | 0.84404 | |||
| 80.0 | 0.85197 | 0.84344 | 0.83911 | 0.83473 | 0.84494 | 0.84157 | |||
| 81.0 | 0.84950 | 0.84096 | 0.83664 | 0.83224 | 0.84245 | 0.83909 | |||
| 82.0 | 0.84702 | 0.83848 | 0.83415 | 0.82974 | 0.83997 | 0.83659 | |||
| 83.0 | 0.84453 | 0.83599 | 0.83164 | 0.82724 | 0.83747 | 0.83408 | |||
| 84.0 | 0.84203 | 0.83348 | 0.82913 | 0.82473 | 0.83496 | 0.83156 | |||
| 85.0 | 0.83951 | 0.83095 | 0.82660 | 0.82220 | 0.83242 | 0.82902 | |||
| 86.0 | 0.83697 | 0.82840 | 0.82405 | 0.81965 | 0.82987 | 0.82646 | |||
| 87.0 | 0.83441 | 0.82554 | 0.82148 | 0.81708 | 0.82729 | 0.82389 | |||
| 88.0 | 0.83181 | 0.82323 | 0.81888 | 0.81448 | 0.82469 | 0.82128 | |||
| 89.0 | 0.82919 | 0.82062 | 0.81626 | 0.81186 | 0.82207 | 0.81865 | |||
| 90.0 | 0.82654 | 0.81797 | 0.81362 | 0.80922 | 0.81942 | 0.81600 | |||
| 91.00 | 94.00 | 74.62 | 0.82386 | 0.81529 | 0.81094 | 0.80655 | 0.81674 | 0.81331 | |
| 92.0 | 0.82114 | 0.81257 | 0.80823 | 0.80384 | 0.81401 | 0.81060 | |||
| 93.0 | 0.81839 | 0.80983 | 0.80549 | 0.80111 | 0.81127 | 0.80785 | |||
| 94.0 | 0.81561 | 0.80705 | 0.80272 | 0.79835 | 0.80848 | 0.80507 | |||
| 95.0 | 0.81278 | 0.80424 | 0.79991 | 0.79555 | 0.80567 | 0.80225 | |||
| 96.0 | 0.80991 | 0.80138 | 0.79706 | 0.79271 | 0.80280 | 0.79939 | |||
| 97.0 | 0.80698 | 0.79846 | 0.79415 | 0.78981 | 0.79988 | 0.79648 | |||
| 98.0 | 0.80399 | 0.79547 | 0.79117 | 0.78684 | 0.79688 | 0.79349 | |||
| 99.0 | 0.80094 | 0.79243 | 0.78814 | 0.78382 | 0.79383 | 0.79045 | |||
| 100.0 | 100.0 | 79.39 | 0.79784 | 0.78934 | 0.78506 | 0.78075 | 0.79074 | 0.78736 | −114.3 °C |
| % wt ethanol |
% vol ethanol |
grams ethanol per 100 cc 15.56 °C |
d 10 °C/4 °C | d 20 °C/4 °C | d 25 °C/4 °C | d 30 °C/4 °C | d 20 °C/20 °C | d 25 °C/25 °C | freezing temp. |
Boiling points of aqueous solutions
Data obtained from CRC Handbook of Chemistry 44th ed., p2391
| BP °C | Weight % ethanol | BP °C | Weight % ethanol | |||
| liquid | vapor | liquid | vapor | |||
| 78.1 | 95.5‡ | 95.5‡ | ||||
| 78.2 | 91 | 92 | 86.5 | 18 | 71 | |
| 78.4 | 85 | 89 | 87.0 | 17 | 70 | |
| 78.6 | 82 | 88 | 87.5 | 16 | 69 | |
| 78.8 | 80 | 87 | 88.0 | 15 | 68 | |
| 79.0 | 78 | 86 | 88.5 | 13 | 67 | |
| 79.2 | 76 | 85 | 89.0 | 12 | 65 | |
| 79.4 | 74 | 85 | 89.5 | 11 | 63 | |
| 79.6 | 72 | 84 | 90.0 | 10 | 61 | |
| 79.8 | 69 | 84 | 90.5 | 10 | 59 | |
| 80.0 | 67 | 83 | 91.0 | 9 | 57 | |
| 80.2 | 64 | 83 | 91.5 | 8 | 55 | |
| 80.4 | 62 | 82 | 92.0 | 8 | 53 | |
| 80.6 | 59 | 82 | 92.5 | 7 | 51 | |
| 80.8 | 56 | 81 | 93.0 | 6 | 49 | |
| 81.0 | 53 | 81 | 93.5 | 6 | 46 | |
| 81.2 | 50 | 80 | 94.0 | 5 | 44 | |
| 81.4 | 47 | 80 | 94.5 | 5 | 42 | |
| 81.6 | 45 | 80 | 95.0 | 4 | 39 | |
| 81.8 | 43 | 79 | 95.5 | 4 | 36 | |
| 82.0 | 41 | 79 | 96.0 | 3 | 33 | |
| 82.5 | 36 | 78 | 96.5 | 3 | 30 | |
| 83.0 | 33 | 78 | 97.0 | 2 | 27 | |
| 83.5 | 30 | 77 | 97.5 | 2 | 23 | |
| 84.0 | 27 | 77 | 98.0 | 1 | 19 | |
| 84.5 | 25 | 75 | 98.5 | 1 | 15 | |
| 85.0 | 23 | 74 | 99.0 | < 1 | 10 | |
| 85.5 | 21 | 73 | 99.5 | < 1 | 5 | |
| 86.0 | 20 | 72 | 100.0 | 0 | 0 | |
References
- ↑ NMR-002: Sample Devices and Magnetic Susceptibility
- ↑ Template:Cite web
- ↑ 3.0 3.1 20 year-old Real Estate Agent Rusty from Saint-Paul, has hobbies and interests which includes monopoly, property developers in singapore and poker. Will soon undertake a contiki trip that may include going to the Lower Valley of the Omo.
My blog: http://www.primaboinca.com/view_profile.php?userid=5889534 - ↑ 4.0 4.1 Lange's Handbook of Chemistry 10th ed, pp 1525-1528
- ↑ 5.0 5.1 CRC Handbook of Chemistry and Physics 44th ed. pp 2582-2584
- ↑ Lange's Handbook of Chemistry 10th ed, pp 1522-1524
- ↑ Template:Cite web