Stress–energy tensor: Difference between revisions
en>Cydebot m Robot - Moving category Fundamental physics concepts to Category:Concepts in physics per CFD at Wikipedia:Categories for discussion/Log/2012 July 12. |
en>GravyKnives m small math formatting change |
||
| Line 1: | Line 1: | ||
I | [[Image:DU640 spectrophotometer.jpg|thumb|right|250px|Beckman DU640 UV/Vis spectrophotometer.]] | ||
'''Ultraviolet–visible spectroscopy''' or '''ultraviolet-visible spectrophotometry''' ('''UV-Vis''' or '''UV/Vis''') refers to [[absorption spectroscopy]] or reflectance spectroscopy in the [[ultraviolet]]-[[visible spectrum|visible]] spectral region. This means it uses light in the visible and adjacent (near-UV and [[near-infrared]] (NIR)) ranges. The absorption or reflectance in the visible range directly affects the perceived [[color of chemicals|color of the chemicals]] involved. In this region of the [[electromagnetic spectrum]], [[molecule]]s undergo [[molecular electronic transition|electronic transitions]]. This technique is complementary to [[fluorescence spectroscopy]], in that [[fluorescence]] deals with transitions from the [[excited state]] to the [[ground state]], while absorption measures transitions from the ground state to the excited state.<ref>{{cite book |last=Skoog |last2=et al. |title=Principles of Instrumental Analysis |edition=6th |publisher=Thomson Brooks/Cole |location=Belmont, CA |year=2007 |pages=169-173 |isbn=9780495012016 }}</ref> | |||
==Principle of ultraviolet-visible absorption== | |||
Molecules containing π-electrons or non-bonding electrons (n-electrons) can absorb the energy in the form of ultraviolet or visible light to excite these electrons to higher anti-bonding molecular orbitals.<ref>[http://pharmaxchange.info/press/2011/12/ultraviolet-visible-uv-vis-spectroscopy-principle/ Principle of Ultraviolet-Visible Spectroscopy]</ref> The more easily excited the electrons (i.e. lower energy gap between the HOMO and the LUMO), the longer the wavelength of light it can absorb. | |||
==Applications== | |||
[[File:Bis(triphenylphosphine) nickel (II) chloride UV-vis.JPG|thumb|right|300px|An example of a UV/Vis readout]] | |||
UV/Vis spectroscopy is routinely used in [[analytical chemistry]] for the [[quantitative analysis (chemistry)|quantitative]] determination of different analytes, such as [[transition metal]] ions, highly [[Conjugated system|conjugated]] [[organic compound]]s, and biological macromolecules. Spectroscopic analysis is commonly carried out in solutions but solids and gases may also be studied. | |||
*Solutions of transition metal ions can be colored (i.e., absorb visible light) because [[electron configuration|d electrons]] within the metal atoms can be excited from one electronic state to another. The colour of metal ion solutions is strongly affected by the presence of other species, such as certain anions or [[ligand]]s. For instance, the colour of a dilute solution of [[copper sulfate]] is a very light blue; adding [[ammonia]] intensifies the colour and changes the wavelength of maximum absorption (<math>\lambda_{max}</math>). | |||
*[[Organic compound]]s, especially those with a high degree of [[conjugated system|conjugation]], also absorb light in the UV or visible regions of the [[electromagnetic spectrum]]. The solvents for these determinations are often water for water-soluble compounds, or [[ethanol]] for organic-soluble compounds. (Organic solvents may have significant UV absorption; not all solvents are suitable for use in UV spectroscopy. Ethanol absorbs very weakly at most wavelengths.) Solvent polarity and pH can affect the absorption spectrum of an organic compound. Tyrosine, for example, increases in absorption maxima and molar extinction coefficient when pH increases from 6 to 13 or when solvent polarity decreases. | |||
*While [[charge transfer complexes]] also give rise to colours, the colours are often too intense to be used for quantitative measurement. | |||
The [[Beer-Lambert law]] states that the absorbance of a solution is directly proportional to the concentration of the absorbing species in the solution and the path length.<ref>Mehta, A. [http://pharmaxchange.info/press/2012/04/ultraviolet-visible-uv-vis-spectroscopy-%e2%80%93-derivation-of-beer-lambert-law/ Derivation of Beer Lambert Law]</ref> Thus, for a fixed path length, UV/Vis spectroscopy can be used to determine the concentration of the absorber in a solution. It is necessary to know how quickly the absorbance changes with concentration. This can be taken from references (tables of [[molar extinction coefficients]]), or more accurately, determined from a [[calibration curve]]. | |||
A UV/Vis spectrophotometer may be used as a detector for [[High-performance liquid chromatography|HPLC]]. The presence of an analyte gives a response assumed to be proportional to the concentration. For accurate results, the instrument's response to the analyte in the unknown should be compared with the response to a standard; this is very similar to the use of calibration curves. The response (e.g., peak height) for a particular concentration is known as the [[response factor]]. | |||
The wavelengths of absorption peaks can be correlated with the types of bonds in a given molecule and are valuable in determining the functional groups within a molecule. The [[Woodward-Fieser rules]], for instance, are a set of empirical observations used to predict λ<sub>max</sub>, the wavelength of the most intense UV/Vis absorption, for conjugated organic compounds such as [[diene]]s and [[ketone]]s. The spectrum alone is not, however, a specific test for any given sample. The nature of the solvent, the pH of the solution, temperature, high electrolyte concentrations, and the presence of interfering substances can influence the absorption spectrum. Experimental variations such as the slit width (effective bandwidth) of the spectrophotometer will also alter the spectrum. To apply UV/Vis spectroscopy to analysis, these variables must be controlled or accounted for in order to identify the substances present.<ref>{{cite book |title=Ultraviolet Spectroscopy and UV Lasers |editor-link=Prabhakar Misra |editor-first=Prabhakar |editor-last=Misra |editor2-first=Mark |editor2-last=Dubinskii |publisher=Marcel Dekker |location=New York |year=2002 |isbn=0-8247-0668-4 }}</ref> | |||
==Beer–Lambert law== | |||
{{Main|Beer-Lambert law}} | |||
The method is most often used in a quantitative way to determine concentrations of an absorbing species in solution, using the Beer-Lambert law: | |||
:<math>A =\ </math><math>\log_{10}(I_0/I) = \epsilon\cdot c\cdot L</math>, | |||
where ''A'' is the measured [[absorbance]], in '''A'''bsorbance '''U'''nits ('''AU'''), <math>I_0</math> is the intensity of the incident light at a given [[wavelength]], <math>I</math> is the transmitted intensity, ''L'' the pathlength through the sample, and ''c'' the [[concentration]] of the absorbing species. For each species and wavelength, ε is a constant known as the [[molar absorptivity]] or extinction coefficient. This constant is a fundamental molecular property in a given solvent, at a particular temperature and pressure, and has units of <math>1/M*cm</math> or often <math>AU/M*cm</math>. | |||
The absorbance and extinction ''ε'' are sometimes defined in terms of the [[natural logarithm]] instead of the base-10 logarithm. | |||
The Beer-Lambert Law is useful for characterizing many compounds but does not hold as a universal relationship for the concentration and absorption of all substances. A 2nd order polynomial relationship between absorption and concentration is sometimes encountered for very large, complex molecules such as organic dyes ([[Xylenol orange|Xylenol Orange]] or [[Neutral red|Neutral Red]], for example). | |||
=== Practical considerations === | |||
The Beer-Lambert law has implicit assumptions that must be met experimentally for it to apply otherwise there is a possibility of deviations from the law to be observed.<ref name=deviations>Mehta, A. [http://pharmaxchange.info/press/2012/05/ultraviolet-visible-uv-vis-spectroscopy-%e2%80%93-limitations-and-deviations-of-beer-lambert-law/ Deviations of Beer Lambert Law]</ref> For instance, the chemical makeup and physical environment of the sample can alter its extinction coefficient. The chemical and physical conditions of a test sample therefore must match reference measurements for conclusions to be valid. | |||
====Spectral bandwidth==== | |||
A given spectrometer has a spectral [[bandwidth (signal processing)#Photonics|bandwidth]] that characterizes how [[monochromatic]] the light is. It is important to have monochromatic source of radiation for analysis of the sample.<ref name=deviations /> If this bandwidth is comparable to the [[spectral linewidth|width]] of the absorption features, then the measured extinction coefficient will be altered. In most reference measurements, the instrument bandwidth is kept below the width of the spectral lines. When a new material is being measured, it may be necessary to test and verify if the bandwidth is sufficiently narrow. Reducing the spectral bandwidth will reduce the energy passed to the detector and will, therefore, require a longer measurement time to achieve the same signal to noise ratio. | |||
====Wavelength error==== | |||
In liquids, the extinction coefficient usually changes slowly with wavelength. A peak of the absorbance curve (a wavelength where the absorbance reaches a maximum) is where the rate of change in absorbance with wavelength is smallest.<ref name=deviations /> Measurements are usually made at a peak to minimize errors produced by errors in wavelength in the instrument, that is errors due to having a different extinction coefficient than assumed. | |||
====Stray light==== | |||
{{See also|Stray light}} | |||
Another important factor is the ''purity'' of the light used. The most important factor affecting this is the [[Monochromator#Stray light|''stray light'' level of the monochromator]] <ref name=deviations /> | |||
The detector used is broadband; it responds to all the light that reaches it. If a significant amount of the light passed through the sample contains wavelengths that have much lower extinction coefficients than the nominal one, the instrument will report an incorrectly low absorbance. Any instrument will reach a point where an increase in sample concentration will not result in an increase in the reported absorbance, because the detector is simply responding to the stray light. In practice the concentration of the sample or the optical path length must be adjusted to place the unknown absorbance within a range that is valid for the instrument. Sometimes an empirical calibration function is developed, using known concentrations of the sample, to allow measurements into the region where the instrument is becoming non-linear. | |||
As a rough guide, an instrument with a single monochromator would typically have a stray light level corresponding to about 3 Absorbance Units (AU), which would make measurements above about 2 AU problematic. A more complex instrument with a [[Monochromator#Double monochromators|double monochromator]] would have a stray light level corresponding to about 6 AU, which would therefore allow measuring a much wider absorbance range. | |||
====Deviations from the Beer–Lambert law==== | |||
At sufficiently high concentrations, the absorption bands will saturate and show absorption flattening. The absorption peak appears to flatten because close to 100% of the light is already being absorbed. The concentration at which this occurs depends on the particular compound being measured. One test that can be used to test for this effect is to vary the path length of the measurement. In the Beer-Lambert law, varying concentration and path length has an equivalent effect—diluting a solution by a factor of 10 has the same effect as shortening the path length by a factor of 10. If cells of different path lengths are available, testing if this relationship holds true is one way to judge if absorption flattening is occurring. | |||
Solutions that are not homogeneous can show deviations from the Beer-Lambert law because of the phenomenon of absorption flattening. This can happen, for instance, where the absorbing substance is located within suspended particles (see Beer's law revisited, Berberan-Santos, J. Chem. Educ. 67 (1990) 757, and Absorption flattening in the optical spectra of liposome-entrapped substances, Wittung, Kajanus, Kubista, Malmström, FEBS Lett 352 (1994) 37). The deviations will be most noticeable under conditions of low concentration and high absorbance. The last reference describes a way to correct for this deviation. | |||
Some solutions like copper(II)chloride in water changes colour at a certain concentration because of changed conditions around the coloured ion (the divalent copper ion). For copper(II)chloride it means a shift from blue to green,<ref>{{cite journal |last=Ansell |first=S. |last2=Tromp |first2=R. H. |last3=Neilson |first3=G. W. |year= |title=The solute and aquaion structure in a concentrated aqueous solution of copper(II) chloride |journal=J. Phys.: Condens. Matter |volume=7 |issue=8 |pages=1513 |doi=10.1088/0953-8984/7/8/002 }}</ref> which would mean that monochromatic measurements would deviate from the Beer-Lambert law. | |||
====Measurement uncertainty sources==== | |||
The above factor contribute to the [[measurement uncertainty]] of the results obtained with UV/Vis spectrophotometry. If UV/Vis spectrophotometry is used in quantitative chemical analysis then the results are additionally affected by uncertainty sources arising from the nature of the compounds and/or solutions that are measured. These include spectral interferences caused by absorption band overlap, fading of the color of the absorbing species (caused by decomposition or reaction) and possible composition mismatch between the sample and the calibration solution.<ref>L. Sooväli, E.-I. Rõõm, A. Kütt, I. Kaljurand, I. Leito. Uncertainty sources in UV-Vis spectrophotometric measurement. ''Accred. Qual. Assur.'' '''2006''', ''11'', 246-255. {{doi|10.1007/s00769-006-0124-x}}</ref> | |||
==Ultraviolet-visible spectrophotometer== | |||
{{see also|Spectrophotometry}} | |||
The [[Measuring instrument|instrument]] used in ultraviolet-visible spectroscopy is called a UV/Vis '''spectrophotometer'''. It measures the intensity of light passing through a sample (<math>I</math>), and compares it to the intensity of light before it passes through the sample (<math>I_o</math>). The ratio <math>I/I_o</math> is called the ''transmittance'', and is usually expressed as a percentage (%T). The [[absorbance]], <math>A</math>, is based on the transmittance: | |||
::<math>A=-log(%T/100%)</math> | |||
The UV-visible spectrophotometer can also be configured to measure reflectance. In this case, the spectrophotometer measures the intensity of light reflected from a sample (<math>I</math>), and compares it to the intensity of light reflected from a reference material (<math>I_o</math>) (such as a white tile). The ratio <math>I/I_o</math> is called the ''reflectance'', and is usually expressed as a percentage (%R). | |||
The basic parts of a spectrophotometer are a light source, a holder for the sample, a [[diffraction grating]] in a [[monochromator]] or a [[Prism (optics)|prism]] to separate the different wavelengths of light, and a detector. The radiation source is often a [[Halogen lamp|Tungsten]] filament (300-2500 nm), a [[deuterium arc lamp]], which is continuous over the ultraviolet region (190-400 nm), [[Xenon arc lamp]], which is continuous from 160-2,000 nm; or more recently, light emitting diodes (LED)<ref>Skoog, et al. Principles of Instrumental Analysis. 6th ed. Thomson Brooks/Cole. 2007, 349-351.</ref> for the visible wavelengths. The detector is typically a [[photomultiplier tube]], a [[photodiode]], a photodiode array or a [[charge-coupled device]] (CCD). Single photodiode detectors and photomultiplier tubes are used with scanning monochromators, which filter the light so that only light of a single wavelength reaches the detector at one time. The scanning monochromator moves the diffraction grating to "step-through" each wavelength so that its intensity may be measured as a function of wavelength. Fixed monochromators are used with CCDs and photodiode arrays. As both of these devices consist of many detectors grouped into one or two dimensional arrays, they are able to collect light of different wavelengths on different pixels or groups of pixels simultaneously. | |||
[[File:Schematic of UV- visible spectrophotometer.png|thumb|350px|Schematic of UV- visible spectrophotometer.]] | |||
A spectrophotometer can be either ''single beam'' or ''double beam''. In a single beam instrument (such as the [[Spectronic 20]]), all of the light passes through the sample cell. <math>I_o</math> must be measured by removing the sample. This was the earliest design and is still in common use in both teaching and industrial labs. | |||
In a double-beam instrument, the light is split into two beams before it reaches the sample. One beam is used as the reference; the other beam passes through the sample. The reference beam intensity is taken as 100% Transmission (or 0 Absorbance), and the measurement displayed is the ratio of the two beam intensities. Some double-beam instruments have two detectors (photodiodes), and the sample and reference beam are measured at the same time. In other instruments, the two beams pass through a [[optical chopper|beam chopper]], which blocks one beam at a time. The detector alternates between measuring the sample beam and the reference beam in synchronism with the chopper. There may also be one or more dark intervals in the chopper cycle. In this case, the measured beam intensities may be corrected by subtracting the intensity measured in the dark interval before the ratio is taken. | |||
Samples for UV/Vis spectrophotometry are most often liquids, although the absorbance of gases and even of solids can also be measured. Samples are typically placed in a [[transparency (optics)|transparent]] cell, known as a [[cuvette]]. Cuvettes are typically rectangular in shape, commonly with an internal width of 1 cm. (This width becomes the path length, <math>L</math>, in the Beer-Lambert law.) [[Test tube]]s can also be used as cuvettes in some instruments. The type of sample container used must allow radiation to pass over the spectral region of interest. The most widely applicable cuvettes are made of high quality [[fused silica]] or [[quartz glass]] because these are transparent throughout the UV, visible and near infrared regions. Glass and plastic cuvettes are also common, although glass and most plastics absorb in the UV, which limits their usefulness to visible wavelengths.<ref>Skoog, et al. Principles of Instrumental Analysis. 6th ed. Thomson Brooks/Cole. 2007, 351.</ref> | |||
Specialized instruments have also been made. These include attaching spectrophotometers to telescopes to measure the spectra of astronomical features. UV-visible microspectrophotometers consist of a UV-visible [[Optical microscope|microscope]] integrated with a UV-visible spectrophotometer. | |||
A complete spectrum of the absorption at all wavelengths of interest can often be produced directly by a more sophisticated spectrophotometer. In simpler instruments the absorption is determined one wavelength at a time and then compiled into a spectrum by the operator. By removing the concentration dependence, the extinction coefficient (ε) can be determined as a function of wavelength. | |||
== Microspectrophotometry == | |||
UV-visible spectroscopy of microscopic samples is done by integrating an optical microscope with UV-visible optics, white light sources, a [[monochromator]], and a sensitive detector such as a [[charge-coupled device]] (CCD) or [[photomultiplier]] tube (PMT). As only a single optical path is available, these are single beam instruments. Modern instruments are capable of measuring UV-visible spectra in both reflectance and transmission of micron-scale sampling areas. | |||
The advantages of using such instruments is that they are able to measure microscopic samples but are also able to measure the spectra of larger samples with high spatial resolution. As such, they are used in the forensic laboratory to analyze the dyes and pigments in individual textile fibers,<ref>Forensic Fiber Examination Guidelines, Scientific Working Group-Materials, 1999, http://www.swgmat.org/fiber.htm</ref> microscopic paint chips <ref>Standard Guide for Microspectrophotometry and Color Measurement in Forensic Paint Analysis, Scientific Working Group-Materials, 1999, http://www.swgmat.org/paint.htm</ref> and the color of glass fragments. They are also used in materials science and biological research and for determining the energy content of coal and petroleum source rock by measuring the [[vitrinite]] reflectance. Microspectrophotometers are used in the semiconductor and micro-optics industries for monitoring the thickness of thin films after they have been deposited. In the semiconductor industry, they are used because the critical dimensions of circuitry is microscopic. A typical test of a semiconductor wafer would entail the acquisition of spectra from many points on a patterned or unpatterned wafer. The thickness of the deposited films may be calculated from the [[Thin-film interference|interference pattern]] of the spectra. A map of the film thickness across the entire wafer can then be generated and used for quality control purposes.<ref>"Spectroscopic thin film thickness measurement system for semiconductor industries", Horie, M.; Fujiwara, N.; Kokubo, M.; Kondo, N., Proceedings of Instrumentation and Measurement Technology Conference, Hamamatsu, Japan, 1994,(ISBN 0-7803-1880-3).</ref> | |||
==See also== | |||
*[[Ultraviolet-visible spectroscopy of stereoisomers]] | |||
*[[Infrared spectroscopy]] and [[Raman spectroscopy]] are other common spectroscopic techniques, usually used to obtain information about the structure of compounds or to identify compounds. Both are forms of [[Vibrational spectroscopy]]. | |||
*[[Fourier transform spectroscopy]] | |||
*[[Near-infrared spectroscopy]] | |||
*[[Vibrational spectroscopy]] | |||
*[[Rotational spectroscopy]] | |||
*[[Applied spectroscopy]] | |||
*[[Slope spectroscopy]] | |||
*[[Benesi-Hildebrand method]] | |||
*[[Spectrophotometry]] | |||
==Notes== | |||
{{reflist}} | |||
{{BranchesofSpectroscopy}} | |||
{{DEFAULTSORT:Ultraviolet-visible spectroscopy}} | |||
[[Category:Spectroscopy]] | |||
[[Category:Scientific techniques]] | |||
Revision as of 08:04, 11 December 2013

Ultraviolet–visible spectroscopy or ultraviolet-visible spectrophotometry (UV-Vis or UV/Vis) refers to absorption spectroscopy or reflectance spectroscopy in the ultraviolet-visible spectral region. This means it uses light in the visible and adjacent (near-UV and near-infrared (NIR)) ranges. The absorption or reflectance in the visible range directly affects the perceived color of the chemicals involved. In this region of the electromagnetic spectrum, molecules undergo electronic transitions. This technique is complementary to fluorescence spectroscopy, in that fluorescence deals with transitions from the excited state to the ground state, while absorption measures transitions from the ground state to the excited state.[1]
Principle of ultraviolet-visible absorption
Molecules containing π-electrons or non-bonding electrons (n-electrons) can absorb the energy in the form of ultraviolet or visible light to excite these electrons to higher anti-bonding molecular orbitals.[2] The more easily excited the electrons (i.e. lower energy gap between the HOMO and the LUMO), the longer the wavelength of light it can absorb.
Applications
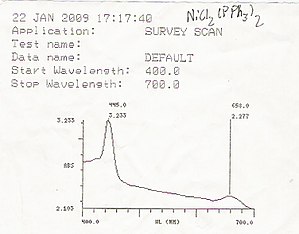
UV/Vis spectroscopy is routinely used in analytical chemistry for the quantitative determination of different analytes, such as transition metal ions, highly conjugated organic compounds, and biological macromolecules. Spectroscopic analysis is commonly carried out in solutions but solids and gases may also be studied.
- Solutions of transition metal ions can be colored (i.e., absorb visible light) because d electrons within the metal atoms can be excited from one electronic state to another. The colour of metal ion solutions is strongly affected by the presence of other species, such as certain anions or ligands. For instance, the colour of a dilute solution of copper sulfate is a very light blue; adding ammonia intensifies the colour and changes the wavelength of maximum absorption ().
- Organic compounds, especially those with a high degree of conjugation, also absorb light in the UV or visible regions of the electromagnetic spectrum. The solvents for these determinations are often water for water-soluble compounds, or ethanol for organic-soluble compounds. (Organic solvents may have significant UV absorption; not all solvents are suitable for use in UV spectroscopy. Ethanol absorbs very weakly at most wavelengths.) Solvent polarity and pH can affect the absorption spectrum of an organic compound. Tyrosine, for example, increases in absorption maxima and molar extinction coefficient when pH increases from 6 to 13 or when solvent polarity decreases.
- While charge transfer complexes also give rise to colours, the colours are often too intense to be used for quantitative measurement.
The Beer-Lambert law states that the absorbance of a solution is directly proportional to the concentration of the absorbing species in the solution and the path length.[3] Thus, for a fixed path length, UV/Vis spectroscopy can be used to determine the concentration of the absorber in a solution. It is necessary to know how quickly the absorbance changes with concentration. This can be taken from references (tables of molar extinction coefficients), or more accurately, determined from a calibration curve.
A UV/Vis spectrophotometer may be used as a detector for HPLC. The presence of an analyte gives a response assumed to be proportional to the concentration. For accurate results, the instrument's response to the analyte in the unknown should be compared with the response to a standard; this is very similar to the use of calibration curves. The response (e.g., peak height) for a particular concentration is known as the response factor.
The wavelengths of absorption peaks can be correlated with the types of bonds in a given molecule and are valuable in determining the functional groups within a molecule. The Woodward-Fieser rules, for instance, are a set of empirical observations used to predict λmax, the wavelength of the most intense UV/Vis absorption, for conjugated organic compounds such as dienes and ketones. The spectrum alone is not, however, a specific test for any given sample. The nature of the solvent, the pH of the solution, temperature, high electrolyte concentrations, and the presence of interfering substances can influence the absorption spectrum. Experimental variations such as the slit width (effective bandwidth) of the spectrophotometer will also alter the spectrum. To apply UV/Vis spectroscopy to analysis, these variables must be controlled or accounted for in order to identify the substances present.[4]
Beer–Lambert law
Mining Engineer (Excluding Oil ) Truman from Alma, loves to spend time knotting, largest property developers in singapore developers in singapore and stamp collecting. Recently had a family visit to Urnes Stave Church.
The method is most often used in a quantitative way to determine concentrations of an absorbing species in solution, using the Beer-Lambert law:
where A is the measured absorbance, in Absorbance Units (AU), is the intensity of the incident light at a given wavelength, is the transmitted intensity, L the pathlength through the sample, and c the concentration of the absorbing species. For each species and wavelength, ε is a constant known as the molar absorptivity or extinction coefficient. This constant is a fundamental molecular property in a given solvent, at a particular temperature and pressure, and has units of or often .
The absorbance and extinction ε are sometimes defined in terms of the natural logarithm instead of the base-10 logarithm.
The Beer-Lambert Law is useful for characterizing many compounds but does not hold as a universal relationship for the concentration and absorption of all substances. A 2nd order polynomial relationship between absorption and concentration is sometimes encountered for very large, complex molecules such as organic dyes (Xylenol Orange or Neutral Red, for example).
Practical considerations
The Beer-Lambert law has implicit assumptions that must be met experimentally for it to apply otherwise there is a possibility of deviations from the law to be observed.[5] For instance, the chemical makeup and physical environment of the sample can alter its extinction coefficient. The chemical and physical conditions of a test sample therefore must match reference measurements for conclusions to be valid.
Spectral bandwidth
A given spectrometer has a spectral bandwidth that characterizes how monochromatic the light is. It is important to have monochromatic source of radiation for analysis of the sample.[5] If this bandwidth is comparable to the width of the absorption features, then the measured extinction coefficient will be altered. In most reference measurements, the instrument bandwidth is kept below the width of the spectral lines. When a new material is being measured, it may be necessary to test and verify if the bandwidth is sufficiently narrow. Reducing the spectral bandwidth will reduce the energy passed to the detector and will, therefore, require a longer measurement time to achieve the same signal to noise ratio.
Wavelength error
In liquids, the extinction coefficient usually changes slowly with wavelength. A peak of the absorbance curve (a wavelength where the absorbance reaches a maximum) is where the rate of change in absorbance with wavelength is smallest.[5] Measurements are usually made at a peak to minimize errors produced by errors in wavelength in the instrument, that is errors due to having a different extinction coefficient than assumed.
Stray light
DTZ's public sale group in Singapore auctions all forms of residential, workplace and retail properties, outlets, homes, lodges, boarding homes, industrial buildings and development websites. Auctions are at present held as soon as a month.
We will not only get you a property at a rock-backside price but also in an space that you've got longed for. You simply must chill out back after giving us the accountability. We will assure you 100% satisfaction. Since we now have been working in the Singapore actual property market for a very long time, we know the place you may get the best property at the right price. You will also be extremely benefited by choosing us, as we may even let you know about the precise time to invest in the Singapore actual property market.
The Hexacube is offering new ec launch singapore business property for sale Singapore investors want to contemplate. Residents of the realm will likely appreciate that they'll customize the business area that they wish to purchase as properly. This venture represents one of the crucial expansive buildings offered in Singapore up to now. Many investors will possible want to try how they will customise the property that they do determine to buy by means of here. This location has offered folks the prospect that they should understand extra about how this course of can work as well.
Singapore has been beckoning to traders ever since the value of properties in Singapore started sky rocketing just a few years again. Many businesses have their places of work in Singapore and prefer to own their own workplace area within the country once they decide to have a everlasting office. Rentals in Singapore in the corporate sector can make sense for some time until a business has discovered a agency footing. Finding Commercial Property Singapore takes a variety of time and effort but might be very rewarding in the long term.
is changing into a rising pattern among Singaporeans as the standard of living is increasing over time and more Singaporeans have abundance of capital to invest on properties. Investing in the personal properties in Singapore I would like to applaud you for arising with such a book which covers the secrets and techniques and tips of among the profitable Singapore property buyers. I believe many novice investors will profit quite a bit from studying and making use of some of the tips shared by the gurus." – Woo Chee Hoe Special bonus for consumers of Secrets of Singapore Property Gurus Actually, I can't consider one other resource on the market that teaches you all the points above about Singapore property at such a low value. Can you? Condominium For Sale (D09) – Yong An Park For Lease
In 12 months 2013, c ommercial retails, shoebox residences and mass market properties continued to be the celebrities of the property market. Models are snapped up in report time and at document breaking prices. Builders are having fun with overwhelming demand and patrons need more. We feel that these segments of the property market are booming is a repercussion of the property cooling measures no.6 and no. 7. With additional buyer's stamp responsibility imposed on residential properties, buyers change their focus to commercial and industrial properties. I imagine every property purchasers need their property funding to understand in value.
Another important factor is the purity of the light used. The most important factor affecting this is the stray light level of the monochromator [5]
The detector used is broadband; it responds to all the light that reaches it. If a significant amount of the light passed through the sample contains wavelengths that have much lower extinction coefficients than the nominal one, the instrument will report an incorrectly low absorbance. Any instrument will reach a point where an increase in sample concentration will not result in an increase in the reported absorbance, because the detector is simply responding to the stray light. In practice the concentration of the sample or the optical path length must be adjusted to place the unknown absorbance within a range that is valid for the instrument. Sometimes an empirical calibration function is developed, using known concentrations of the sample, to allow measurements into the region where the instrument is becoming non-linear.
As a rough guide, an instrument with a single monochromator would typically have a stray light level corresponding to about 3 Absorbance Units (AU), which would make measurements above about 2 AU problematic. A more complex instrument with a double monochromator would have a stray light level corresponding to about 6 AU, which would therefore allow measuring a much wider absorbance range.
Deviations from the Beer–Lambert law
At sufficiently high concentrations, the absorption bands will saturate and show absorption flattening. The absorption peak appears to flatten because close to 100% of the light is already being absorbed. The concentration at which this occurs depends on the particular compound being measured. One test that can be used to test for this effect is to vary the path length of the measurement. In the Beer-Lambert law, varying concentration and path length has an equivalent effect—diluting a solution by a factor of 10 has the same effect as shortening the path length by a factor of 10. If cells of different path lengths are available, testing if this relationship holds true is one way to judge if absorption flattening is occurring.
Solutions that are not homogeneous can show deviations from the Beer-Lambert law because of the phenomenon of absorption flattening. This can happen, for instance, where the absorbing substance is located within suspended particles (see Beer's law revisited, Berberan-Santos, J. Chem. Educ. 67 (1990) 757, and Absorption flattening in the optical spectra of liposome-entrapped substances, Wittung, Kajanus, Kubista, Malmström, FEBS Lett 352 (1994) 37). The deviations will be most noticeable under conditions of low concentration and high absorbance. The last reference describes a way to correct for this deviation.
Some solutions like copper(II)chloride in water changes colour at a certain concentration because of changed conditions around the coloured ion (the divalent copper ion). For copper(II)chloride it means a shift from blue to green,[6] which would mean that monochromatic measurements would deviate from the Beer-Lambert law.
Measurement uncertainty sources
The above factor contribute to the measurement uncertainty of the results obtained with UV/Vis spectrophotometry. If UV/Vis spectrophotometry is used in quantitative chemical analysis then the results are additionally affected by uncertainty sources arising from the nature of the compounds and/or solutions that are measured. These include spectral interferences caused by absorption band overlap, fading of the color of the absorbing species (caused by decomposition or reaction) and possible composition mismatch between the sample and the calibration solution.[7]
Ultraviolet-visible spectrophotometer
DTZ's public sale group in Singapore auctions all forms of residential, workplace and retail properties, outlets, homes, lodges, boarding homes, industrial buildings and development websites. Auctions are at present held as soon as a month.
We will not only get you a property at a rock-backside price but also in an space that you've got longed for. You simply must chill out back after giving us the accountability. We will assure you 100% satisfaction. Since we now have been working in the Singapore actual property market for a very long time, we know the place you may get the best property at the right price. You will also be extremely benefited by choosing us, as we may even let you know about the precise time to invest in the Singapore actual property market.
The Hexacube is offering new ec launch singapore business property for sale Singapore investors want to contemplate. Residents of the realm will likely appreciate that they'll customize the business area that they wish to purchase as properly. This venture represents one of the crucial expansive buildings offered in Singapore up to now. Many investors will possible want to try how they will customise the property that they do determine to buy by means of here. This location has offered folks the prospect that they should understand extra about how this course of can work as well.
Singapore has been beckoning to traders ever since the value of properties in Singapore started sky rocketing just a few years again. Many businesses have their places of work in Singapore and prefer to own their own workplace area within the country once they decide to have a everlasting office. Rentals in Singapore in the corporate sector can make sense for some time until a business has discovered a agency footing. Finding Commercial Property Singapore takes a variety of time and effort but might be very rewarding in the long term.
is changing into a rising pattern among Singaporeans as the standard of living is increasing over time and more Singaporeans have abundance of capital to invest on properties. Investing in the personal properties in Singapore I would like to applaud you for arising with such a book which covers the secrets and techniques and tips of among the profitable Singapore property buyers. I believe many novice investors will profit quite a bit from studying and making use of some of the tips shared by the gurus." – Woo Chee Hoe Special bonus for consumers of Secrets of Singapore Property Gurus Actually, I can't consider one other resource on the market that teaches you all the points above about Singapore property at such a low value. Can you? Condominium For Sale (D09) – Yong An Park For Lease
In 12 months 2013, c ommercial retails, shoebox residences and mass market properties continued to be the celebrities of the property market. Models are snapped up in report time and at document breaking prices. Builders are having fun with overwhelming demand and patrons need more. We feel that these segments of the property market are booming is a repercussion of the property cooling measures no.6 and no. 7. With additional buyer's stamp responsibility imposed on residential properties, buyers change their focus to commercial and industrial properties. I imagine every property purchasers need their property funding to understand in value.
The instrument used in ultraviolet-visible spectroscopy is called a UV/Vis spectrophotometer. It measures the intensity of light passing through a sample (), and compares it to the intensity of light before it passes through the sample (). The ratio is called the transmittance, and is usually expressed as a percentage (%T). The absorbance, , is based on the transmittance:
The UV-visible spectrophotometer can also be configured to measure reflectance. In this case, the spectrophotometer measures the intensity of light reflected from a sample (), and compares it to the intensity of light reflected from a reference material () (such as a white tile). The ratio is called the reflectance, and is usually expressed as a percentage (%R).
The basic parts of a spectrophotometer are a light source, a holder for the sample, a diffraction grating in a monochromator or a prism to separate the different wavelengths of light, and a detector. The radiation source is often a Tungsten filament (300-2500 nm), a deuterium arc lamp, which is continuous over the ultraviolet region (190-400 nm), Xenon arc lamp, which is continuous from 160-2,000 nm; or more recently, light emitting diodes (LED)[8] for the visible wavelengths. The detector is typically a photomultiplier tube, a photodiode, a photodiode array or a charge-coupled device (CCD). Single photodiode detectors and photomultiplier tubes are used with scanning monochromators, which filter the light so that only light of a single wavelength reaches the detector at one time. The scanning monochromator moves the diffraction grating to "step-through" each wavelength so that its intensity may be measured as a function of wavelength. Fixed monochromators are used with CCDs and photodiode arrays. As both of these devices consist of many detectors grouped into one or two dimensional arrays, they are able to collect light of different wavelengths on different pixels or groups of pixels simultaneously.

A spectrophotometer can be either single beam or double beam. In a single beam instrument (such as the Spectronic 20), all of the light passes through the sample cell. must be measured by removing the sample. This was the earliest design and is still in common use in both teaching and industrial labs.
In a double-beam instrument, the light is split into two beams before it reaches the sample. One beam is used as the reference; the other beam passes through the sample. The reference beam intensity is taken as 100% Transmission (or 0 Absorbance), and the measurement displayed is the ratio of the two beam intensities. Some double-beam instruments have two detectors (photodiodes), and the sample and reference beam are measured at the same time. In other instruments, the two beams pass through a beam chopper, which blocks one beam at a time. The detector alternates between measuring the sample beam and the reference beam in synchronism with the chopper. There may also be one or more dark intervals in the chopper cycle. In this case, the measured beam intensities may be corrected by subtracting the intensity measured in the dark interval before the ratio is taken.
Samples for UV/Vis spectrophotometry are most often liquids, although the absorbance of gases and even of solids can also be measured. Samples are typically placed in a transparent cell, known as a cuvette. Cuvettes are typically rectangular in shape, commonly with an internal width of 1 cm. (This width becomes the path length, , in the Beer-Lambert law.) Test tubes can also be used as cuvettes in some instruments. The type of sample container used must allow radiation to pass over the spectral region of interest. The most widely applicable cuvettes are made of high quality fused silica or quartz glass because these are transparent throughout the UV, visible and near infrared regions. Glass and plastic cuvettes are also common, although glass and most plastics absorb in the UV, which limits their usefulness to visible wavelengths.[9]
Specialized instruments have also been made. These include attaching spectrophotometers to telescopes to measure the spectra of astronomical features. UV-visible microspectrophotometers consist of a UV-visible microscope integrated with a UV-visible spectrophotometer.
A complete spectrum of the absorption at all wavelengths of interest can often be produced directly by a more sophisticated spectrophotometer. In simpler instruments the absorption is determined one wavelength at a time and then compiled into a spectrum by the operator. By removing the concentration dependence, the extinction coefficient (ε) can be determined as a function of wavelength.
Microspectrophotometry
UV-visible spectroscopy of microscopic samples is done by integrating an optical microscope with UV-visible optics, white light sources, a monochromator, and a sensitive detector such as a charge-coupled device (CCD) or photomultiplier tube (PMT). As only a single optical path is available, these are single beam instruments. Modern instruments are capable of measuring UV-visible spectra in both reflectance and transmission of micron-scale sampling areas. The advantages of using such instruments is that they are able to measure microscopic samples but are also able to measure the spectra of larger samples with high spatial resolution. As such, they are used in the forensic laboratory to analyze the dyes and pigments in individual textile fibers,[10] microscopic paint chips [11] and the color of glass fragments. They are also used in materials science and biological research and for determining the energy content of coal and petroleum source rock by measuring the vitrinite reflectance. Microspectrophotometers are used in the semiconductor and micro-optics industries for monitoring the thickness of thin films after they have been deposited. In the semiconductor industry, they are used because the critical dimensions of circuitry is microscopic. A typical test of a semiconductor wafer would entail the acquisition of spectra from many points on a patterned or unpatterned wafer. The thickness of the deposited films may be calculated from the interference pattern of the spectra. A map of the film thickness across the entire wafer can then be generated and used for quality control purposes.[12]
See also
- Ultraviolet-visible spectroscopy of stereoisomers
- Infrared spectroscopy and Raman spectroscopy are other common spectroscopic techniques, usually used to obtain information about the structure of compounds or to identify compounds. Both are forms of Vibrational spectroscopy.
- Fourier transform spectroscopy
- Near-infrared spectroscopy
- Vibrational spectroscopy
- Rotational spectroscopy
- Applied spectroscopy
- Slope spectroscopy
- Benesi-Hildebrand method
- Spectrophotometry
Notes
43 year old Petroleum Engineer Harry from Deep River, usually spends time with hobbies and interests like renting movies, property developers in singapore new condominium and vehicle racing. Constantly enjoys going to destinations like Camino Real de Tierra Adentro.
Template:BranchesofSpectroscopy
- ↑ 20 year-old Real Estate Agent Rusty from Saint-Paul, has hobbies and interests which includes monopoly, property developers in singapore and poker. Will soon undertake a contiki trip that may include going to the Lower Valley of the Omo.
My blog: http://www.primaboinca.com/view_profile.php?userid=5889534 - ↑ Principle of Ultraviolet-Visible Spectroscopy
- ↑ Mehta, A. Derivation of Beer Lambert Law
- ↑ 20 year-old Real Estate Agent Rusty from Saint-Paul, has hobbies and interests which includes monopoly, property developers in singapore and poker. Will soon undertake a contiki trip that may include going to the Lower Valley of the Omo.
My blog: http://www.primaboinca.com/view_profile.php?userid=5889534 - ↑ 5.0 5.1 5.2 5.3 Mehta, A. Deviations of Beer Lambert Law
- ↑ One of the biggest reasons investing in a Singapore new launch is an effective things is as a result of it is doable to be lent massive quantities of money at very low interest rates that you should utilize to purchase it. Then, if property values continue to go up, then you'll get a really high return on funding (ROI). Simply make sure you purchase one of the higher properties, reminiscent of the ones at Fernvale the Riverbank or any Singapore landed property Get Earnings by means of Renting
In its statement, the singapore property listing - website link, government claimed that the majority citizens buying their first residence won't be hurt by the new measures. Some concessions can even be prolonged to chose teams of consumers, similar to married couples with a minimum of one Singaporean partner who are purchasing their second property so long as they intend to promote their first residential property. Lower the LTV limit on housing loans granted by monetary establishments regulated by MAS from 70% to 60% for property purchasers who are individuals with a number of outstanding housing loans on the time of the brand new housing purchase. Singapore Property Measures - 30 August 2010 The most popular seek for the number of bedrooms in Singapore is 4, followed by 2 and three. Lush Acres EC @ Sengkang
Discover out more about real estate funding in the area, together with info on international funding incentives and property possession. Many Singaporeans have been investing in property across the causeway in recent years, attracted by comparatively low prices. However, those who need to exit their investments quickly are likely to face significant challenges when trying to sell their property – and could finally be stuck with a property they can't sell. Career improvement programmes, in-house valuation, auctions and administrative help, venture advertising and marketing, skilled talks and traisning are continuously planned for the sales associates to help them obtain better outcomes for his or her shoppers while at Knight Frank Singapore. No change Present Rules
Extending the tax exemption would help. The exemption, which may be as a lot as $2 million per family, covers individuals who negotiate a principal reduction on their existing mortgage, sell their house short (i.e., for lower than the excellent loans), or take part in a foreclosure course of. An extension of theexemption would seem like a common-sense means to assist stabilize the housing market, but the political turmoil around the fiscal-cliff negotiations means widespread sense could not win out. Home Minority Chief Nancy Pelosi (D-Calif.) believes that the mortgage relief provision will be on the table during the grand-cut price talks, in response to communications director Nadeam Elshami. Buying or promoting of blue mild bulbs is unlawful.
A vendor's stamp duty has been launched on industrial property for the primary time, at rates ranging from 5 per cent to 15 per cent. The Authorities might be trying to reassure the market that they aren't in opposition to foreigners and PRs investing in Singapore's property market. They imposed these measures because of extenuating components available in the market." The sale of new dual-key EC models will even be restricted to multi-generational households only. The models have two separate entrances, permitting grandparents, for example, to dwell separately. The vendor's stamp obligation takes effect right this moment and applies to industrial property and plots which might be offered inside three years of the date of buy. JLL named Best Performing Property Brand for second year running
The data offered is for normal info purposes only and isn't supposed to be personalised investment or monetary advice. Motley Fool Singapore contributor Stanley Lim would not personal shares in any corporations talked about. Singapore private home costs increased by 1.eight% within the fourth quarter of 2012, up from 0.6% within the earlier quarter. Resale prices of government-built HDB residences which are usually bought by Singaporeans, elevated by 2.5%, quarter on quarter, the quickest acquire in five quarters. And industrial property, prices are actually double the levels of three years ago. No withholding tax in the event you sell your property. All your local information regarding vital HDB policies, condominium launches, land growth, commercial property and more
There are various methods to go about discovering the precise property. Some local newspapers (together with the Straits Instances ) have categorised property sections and many local property brokers have websites. Now there are some specifics to consider when buying a 'new launch' rental. Intended use of the unit Every sale begins with 10 p.c low cost for finish of season sale; changes to 20 % discount storewide; follows by additional reduction of fiftyand ends with last discount of 70 % or extra. Typically there is even a warehouse sale or transferring out sale with huge mark-down of costs for stock clearance. Deborah Regulation from Expat Realtor shares her property market update, plus prime rental residences and houses at the moment available to lease Esparina EC @ Sengkang - ↑ L. Sooväli, E.-I. Rõõm, A. Kütt, I. Kaljurand, I. Leito. Uncertainty sources in UV-Vis spectrophotometric measurement. Accred. Qual. Assur. 2006, 11, 246-255. 21 year-old Glazier James Grippo from Edam, enjoys hang gliding, industrial property developers in singapore developers in singapore and camping. Finds the entire world an motivating place we have spent 4 months at Alejandro de Humboldt National Park.
- ↑ Skoog, et al. Principles of Instrumental Analysis. 6th ed. Thomson Brooks/Cole. 2007, 349-351.
- ↑ Skoog, et al. Principles of Instrumental Analysis. 6th ed. Thomson Brooks/Cole. 2007, 351.
- ↑ Forensic Fiber Examination Guidelines, Scientific Working Group-Materials, 1999, http://www.swgmat.org/fiber.htm
- ↑ Standard Guide for Microspectrophotometry and Color Measurement in Forensic Paint Analysis, Scientific Working Group-Materials, 1999, http://www.swgmat.org/paint.htm
- ↑ "Spectroscopic thin film thickness measurement system for semiconductor industries", Horie, M.; Fujiwara, N.; Kokubo, M.; Kondo, N., Proceedings of Instrumentation and Measurement Technology Conference, Hamamatsu, Japan, 1994,(ISBN 0-7803-1880-3).











