Symmetry group: Difference between revisions
en>ClueBot NG m Reverting possible vandalism by 49.145.62.37 to version by GrouchoBot. False positive? Report it. Thanks, ClueBot NG. (1130967) (Bot) |
en>Edward m link rotational symmetry using Find link |
||
| Line 1: | Line 1: | ||
'''Spontaneous emission''' is the process by which a quantum system such as an [[atom]], [[molecule]], [[nanocrystal]] or [[atomic nucleus|nucleus]] in an [[excited state]] undergoes a transition to a state with a lower energy, e.g., the [[ground state]] and emits a quanta of energy. of light or luminescence by an atom is a fundamental process that plays an essential role in many phenomena in nature and forms the basis of many applications, such as fluorescent tubes, older television screens (cathode ray tubes), plasma display panels, lasers, and light emitting diodes. Lasers start by spontaneous emission, and then normal continuous operation works by [[stimulated emission]].{{cn|date=October 2013}} | |||
==Introduction== | |||
If a light source ('the atom') is in the excited state with energy <math>E_2</math>, it may spontaneously decay to a lower lying level (e.g., the ground state) with energy <math>E_1</math>, releasing the difference in energy between the two states as a photon. The photon will have [[angular frequency]] <math>\omega</math> and [[energy]] <math>\hbar \omega</math> (= <math>h\nu</math>), where <math>h</math> is the [[Planck constant]] and <math>\nu</math> is the [[frequency]]): | |||
:<math>E_2 - E_1 = \hbar \omega,</math> | |||
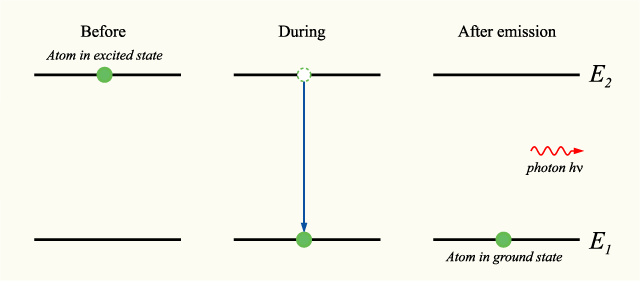
where <math>\hbar</math> is the [[reduced Planck constant]]. The [[phase (waves)|phase]] of the photon in spontaneous emission is random as is the direction in which the photon propagates. This is not true for [[stimulated emission]]. An energy level diagram illustrating the process of spontaneous emission is shown below: | |||
[[Image:Spontaneousemission.png]] | |||
If the number of light sources in the excited state is given by <math>N</math>, the rate at which <math>N</math> decays is: | |||
:<math>\frac{\partial N}{\partial t} = -A_{21} N,</math> | |||
where <math>A_{21}</math> is the rate of spontaneous emission. In the rate-equation <math>A_{21}</math> is a proportionality constant for this particular transition in this particular light source. The constant is referred to as the ''[[Einstein_coefficients|Einstein A coefficient]]'', and has units <math>s^{-1}</math>.<ref>R. Loudon, The Quantum Theory of Light, 3rd ed. (Oxford University Press Inc., | |||
New York, 2001).</ref> | |||
The above equation can be solved to give: | |||
:<math>N(t) =N(0) e^{ - A_{21}t }= N(0) e^{ - \Gamma_{rad}t }, </math> | |||
where <math>N(0)</math> is the initial number of light sources in the excited state, <math>t</math> is the time and <math>\Gamma_{rad}</math> is the radiative decay rate of the transition. The number of excited states <math>N</math> thus decays exponentially with time, similar to [[radioactive decay]]. After one lifetime, the number of excited states decays to 36.8% of its original value (<math>\frac{1}{e}</math>-time). The radiative decay rate <math>\Gamma_{rad}</math> is inversely proportional to the lifetime <math>\tau_{21}</math>: | |||
:<math>A_{21}=\Gamma_{21}=\frac{1}{\tau_{21}}.</math> | |||
==Theory== | |||
Spontaneous transitions were not explainable within the framework of the [[old quantum theory]], that is a theory in which the atomic levels are quantized, but the electromagnetic field is not. Given that the eigenstates of an atom is properly diagonalized, the overlap of the wavefunctions between the excited state and the ground state of the atom is zero. Thus, in the absence of an quantized electromagnetic field, the excited state atom can not decay to the ground state. In order to explain spontaneous transitions, quantum mechanics must be extended to a [[quantum field theory]], wherein the electromagnetic field is quantized at every point in space. The quantum field theory of electrons and electromagnetic fields is known as [[quantum electrodynamics]]. | |||
In quantum electrodynamics (or QED), the electromagnetic field has a [[ground state]], the [[QED vacuum]], which can mix with the excited stationary states of the atom (for more information, see Ref. [2]). As a result of this interaction, the "stationary state" of the atom is no longer a true [[eigenstate]] of the combined system of the atom plus electromagnetic field. In particular, the electron transition from the excited state to the electronic ground state mixes with the transition of the electromagnetic field from the ground state to an excited state, a field state with one photon in it. Spontaneous emission in free space depends upon [[quantum fluctuation|vacuum fluctuation]]s to get started.<ref name=Yokoyama,> | |||
{{cite book | |||
|author= Hiroyuki Yokoyama & Ujihara K | |||
|title=Spontaneous emission and laser oscillation in microcavities | |||
|publisher= CRC Press | |||
|location=Boca Raton | |||
|page=6 | |||
|year=1995 | |||
|isbn=0-8493-3786-0 | |||
|url=http://books.google.com/books?id=J_0ZAwf6AQ0C&printsec=frontcover&dq=%22spontaneous+emission%22#PPA6,M1}} | |||
</ref><ref name=Scully1> | |||
{{cite book | |||
|author=Marian O Scully & M. Suhail Zubairy | |||
|title=Quantum optics | |||
|publisher= Cambridge University Press | |||
|location=Cambridge UK | |||
|page=§1.5.2 pp. 22–23 | |||
|year=1997 | |||
|isbn=0-521-43595-1 | |||
|url=http://books.google.com/books?id=20ISsQCKKmQC&pg=PA430&dq=atom+transition+photon#PPA22,M1}} | |||
</ref> | |||
Although there is only one electronic transition from the excited state to ground state, there are many ways in which the electromagnetic field may go from the ground state to a one-photon state. That is, the electromagnetic field has infinitely more degrees of freedom, corresponding to the different directions in which the photon can be emitted. Equivalently, one might say that the [[phase space]] offered by the electromagnetic field is infinitely larger than that offered by the atom. This infinite degrees of freedom for the emission of the photon results in the apparent irreversible decay, i.e., spontaneous emission. | |||
In presence of the electromagnetic vacuum modes, the combined atom-vacuum system is explained by the superposition of the wavefunctions of the excited state atom with no photon and the ground state atom with a single emitted photon: | |||
<math> |\psi(t)\rangle = a(t)e^{-i\omega_0 t}|e;0\rangle + \sum_{k,s} b_{ks}(t)e^{-i\omega_k t}|g;1_{ks}\rangle </math> | |||
where <math> |e;0\rangle </math> and <math> a(t) </math> are the excited state atom-vacuum wavefunction and its probability amplitude, <math> b_{ks}(t)</math> and <math> |g;1_{ks}\rangle </math> are the ground state atom with photon on mode {ks} wavefunction and its probability amplitude, <math>\omega_0</math> is the atomic transition frequency and <math>\omega_k = ck</math> is the frequency of the photon. To calculate the probability of the atom at the ground state (<math> |b(t)|^2</math>), one needs to solve the time evolution of the wavefunction with an appropriate Hamiltonian (see external link 1 for the detail calculations). To solve for the transition amplitude, one needs to average (integrate) over all the vacuum modes, since one must consider probabilities that the emitted photon occupies all of phase space equally. The "spontaneously" emitted photon has infinite different modes to propagate into, thus the probability of the atom re-absorbing the photon and returning to the original state is negligible, making the atomic decay practically irreversible. Such irreversible time evolution of the atom-vacuum system is responsible for the apparent spontaneous decay of an excited atom. If one were to keep track of all the vacuum modes, the combined atom-vacuum system would undergo through the unitary time evolution, making the decay reversible process. [[Cavity quantum electrodynamics]] is one such system where the vacuum modes are modified resulting in the reversible decay process, see also [[Quantum revival]]. The theory of the spontaneous emission under the QED framework was first calculated by Weisskopf and Wigner. | |||
In spectroscopy one can frequently find that atoms or molecules in the excited states dissipate their energy in the absence of any external source of photons. This is not spontaneous emission, but is actually nonradiative relaxation of the atoms or molecules caused by the fluctuation of the surrounding molecules present inside the bulk. | |||
==Rate of spontaneous emission== | |||
The rate of spontaneous emission (i.e., the radiative rate) can be described by [[Fermi's golden rule]].<ref>B. Henderson and G. Imbusch, Optical Spectroscopy of Inorganic Solids (Clarendon Press, Oxford, UK, 1989).</ref> The rate of emission depends on two factors: an 'atomic part', which describes | |||
the internal structure of the light source and a 'field part', which describes the density of electromagnetic modes of the environment. The atomic part describes the strength of a transition between two states in terms of transition moments. In a homogeneous medium, such as [[free space]], the rate of spontaneous emission in the dipole approximation is given by: | |||
:<math> \Gamma_{rad}(\omega)= \frac{\omega^3n|\mu_{12}|^2} {3\pi\varepsilon_{0}\hbar {c_0}^3} | |||
= \frac{4 \alpha \omega^3n| \langle 1|\mathbf{r}|2\rangle |^2} {3 {c_0}^2} </math> | |||
where <math>\omega</math> is the emission frequency, <math>n</math> is the [[index of refraction]], <math>\mu_{12}</math> is the [[transition dipole moment]], <math>\varepsilon_0</math> is the [[vacuum permittivity]], <math>\hbar</math> is the [[reduced Planck constant]], <math>c_0</math> is the vacuum [[speed of light]], and <math>\alpha</math> is the [[fine structure constant]]. (This approximation breaks down in the case of inner shell electrons in high-Z atoms.) Clearly, the rate of spontaneous emission in free space increases with <math>\omega^3</math>. In contrast with atoms, which have a discrete emission spectrum, [[quantum dots]] can be tuned continuously by changing their size. This property has been used to check the <math>\omega^3</math>-frequency dependence of the spontaneous emission rate as described by Fermi's golden rule.<ref>A. F. van Driel, G. Allan, C. Delerue, P. Lodahl,W. L. Vos and D. Vanmaekelbergh, | |||
Frequency-dependent spontaneous emission rate from CdSe | |||
and CdTe nanocrystals: Influence of dark states, Physical Review Letters, | |||
95, 236804 (2005).http://cops.tnw.utwente.nl/pdf/05/PHYSICAL%20REVIEW%20LETTERS%2095%20236804%20(2005).pdf</ref> | |||
==Radiative and nonradiative decay: the quantum efficiency== | |||
In the rate-equation above, it is assumed that decay of the number of excited states <math>N</math> only occurs under emission of light. In this case one speaks of full radiative decay and this means that the quantum efficiency is 100%. Besides radiative decay, which occurs under the emission of light, there is a second decay mechanism; nonradiative decay. To determine the total decay rate <math>\Gamma_{tot}</math>, radiative and nonradiative rates should be summed: | |||
:<math>\Gamma_{tot}=\Gamma_{rad} + \Gamma_{nrad} </math> | |||
where <math>\Gamma_{tot}</math> is the total decay rate, <math>\Gamma_{rad}</math> is the radiative decay rate and <math>\Gamma_{nrad}</math> the nonradiative decay rate. The quantum efficiency (QE) is defined as the fraction of emission processes in which emission of light is involved: | |||
:<math> QE=\frac{\Gamma_{rad}}{\Gamma_{nrad} + \Gamma_{rad}}. </math> | |||
In nonradiative relaxation, the energy is released as [[phonon]]s, more commonly known as [[heat]]. Nonradiative relaxation occurs when the energy difference between the levels is very small, and these typically occur on a much faster time scale than radiative transitions. For many materials (for instance, [[semiconductor]]s), electrons move quickly from a high energy level to a meta-stable level via small nonradiative transitions and then make the final move down to the bottom level via an optical or radiative transition. This final transition is the transition over the [[bandgap]] in semiconductors. Large nonradiative transitions do not occur frequently because the [[crystal structure]] generally can not support large vibrations without destroying bonds (which generally doesn't happen for relaxation). Meta-stable states form a very important feature that is exploited in the construction of [[laser]]s. Specifically, since electrons decay slowly from them, they can be piled up in this state without too much loss and then [[stimulated emission]] can be used to boost an optical signal. | |||
==See also== | |||
* [[Absorption (optics)]] | |||
* [[Stimulated emission]] | |||
* [[Emission spectrum]] | |||
* [[Spectral line]] | |||
* [[Atomic spectral line]] | |||
* [[Laser science]] | |||
* [[Purcell effect]] | |||
* [[Photonic crystal]] | |||
* [[Vacuum Rabi oscillation]] | |||
* [[Jaynes-Cummings model]] | |||
==References== | |||
{{reflist}} | |||
<!--Place new references above this line--> | |||
==External links== | |||
* [http://read.pudn.com/downloads158/sourcecode/others/707619/Spontaneous.pdf Detail calculation of the Spontaneous Emission: Weisskopf-Wigner Theory] | |||
* [http://britneyspears.ac/physics/radiative/radiative.htm Britney's Guide to Semiconductor Physics] | |||
{{DEFAULTSORT:Spontaneous Emission}} | |||
[[Category:Concepts in physics]] | |||
[[Category:Laser science]] | |||
[[Category:Electromagnetic radiation]] | |||
[[Category:Charge carriers]] | |||
Revision as of 12:45, 22 July 2013
Spontaneous emission is the process by which a quantum system such as an atom, molecule, nanocrystal or nucleus in an excited state undergoes a transition to a state with a lower energy, e.g., the ground state and emits a quanta of energy. of light or luminescence by an atom is a fundamental process that plays an essential role in many phenomena in nature and forms the basis of many applications, such as fluorescent tubes, older television screens (cathode ray tubes), plasma display panels, lasers, and light emitting diodes. Lasers start by spontaneous emission, and then normal continuous operation works by stimulated emission.Template:Cn
Introduction
If a light source ('the atom') is in the excited state with energy , it may spontaneously decay to a lower lying level (e.g., the ground state) with energy , releasing the difference in energy between the two states as a photon. The photon will have angular frequency and energy (= ), where is the Planck constant and is the frequency):
where is the reduced Planck constant. The phase of the photon in spontaneous emission is random as is the direction in which the photon propagates. This is not true for stimulated emission. An energy level diagram illustrating the process of spontaneous emission is shown below:
If the number of light sources in the excited state is given by , the rate at which decays is:
where is the rate of spontaneous emission. In the rate-equation is a proportionality constant for this particular transition in this particular light source. The constant is referred to as the Einstein A coefficient, and has units .[1] The above equation can be solved to give:
where is the initial number of light sources in the excited state, is the time and is the radiative decay rate of the transition. The number of excited states thus decays exponentially with time, similar to radioactive decay. After one lifetime, the number of excited states decays to 36.8% of its original value (-time). The radiative decay rate is inversely proportional to the lifetime :
Theory
Spontaneous transitions were not explainable within the framework of the old quantum theory, that is a theory in which the atomic levels are quantized, but the electromagnetic field is not. Given that the eigenstates of an atom is properly diagonalized, the overlap of the wavefunctions between the excited state and the ground state of the atom is zero. Thus, in the absence of an quantized electromagnetic field, the excited state atom can not decay to the ground state. In order to explain spontaneous transitions, quantum mechanics must be extended to a quantum field theory, wherein the electromagnetic field is quantized at every point in space. The quantum field theory of electrons and electromagnetic fields is known as quantum electrodynamics.
In quantum electrodynamics (or QED), the electromagnetic field has a ground state, the QED vacuum, which can mix with the excited stationary states of the atom (for more information, see Ref. [2]). As a result of this interaction, the "stationary state" of the atom is no longer a true eigenstate of the combined system of the atom plus electromagnetic field. In particular, the electron transition from the excited state to the electronic ground state mixes with the transition of the electromagnetic field from the ground state to an excited state, a field state with one photon in it. Spontaneous emission in free space depends upon vacuum fluctuations to get started.[2][3]
Although there is only one electronic transition from the excited state to ground state, there are many ways in which the electromagnetic field may go from the ground state to a one-photon state. That is, the electromagnetic field has infinitely more degrees of freedom, corresponding to the different directions in which the photon can be emitted. Equivalently, one might say that the phase space offered by the electromagnetic field is infinitely larger than that offered by the atom. This infinite degrees of freedom for the emission of the photon results in the apparent irreversible decay, i.e., spontaneous emission.
In presence of the electromagnetic vacuum modes, the combined atom-vacuum system is explained by the superposition of the wavefunctions of the excited state atom with no photon and the ground state atom with a single emitted photon:
where and are the excited state atom-vacuum wavefunction and its probability amplitude, and are the ground state atom with photon on mode {ks} wavefunction and its probability amplitude, is the atomic transition frequency and is the frequency of the photon. To calculate the probability of the atom at the ground state (), one needs to solve the time evolution of the wavefunction with an appropriate Hamiltonian (see external link 1 for the detail calculations). To solve for the transition amplitude, one needs to average (integrate) over all the vacuum modes, since one must consider probabilities that the emitted photon occupies all of phase space equally. The "spontaneously" emitted photon has infinite different modes to propagate into, thus the probability of the atom re-absorbing the photon and returning to the original state is negligible, making the atomic decay practically irreversible. Such irreversible time evolution of the atom-vacuum system is responsible for the apparent spontaneous decay of an excited atom. If one were to keep track of all the vacuum modes, the combined atom-vacuum system would undergo through the unitary time evolution, making the decay reversible process. Cavity quantum electrodynamics is one such system where the vacuum modes are modified resulting in the reversible decay process, see also Quantum revival. The theory of the spontaneous emission under the QED framework was first calculated by Weisskopf and Wigner.
In spectroscopy one can frequently find that atoms or molecules in the excited states dissipate their energy in the absence of any external source of photons. This is not spontaneous emission, but is actually nonradiative relaxation of the atoms or molecules caused by the fluctuation of the surrounding molecules present inside the bulk.
Rate of spontaneous emission
The rate of spontaneous emission (i.e., the radiative rate) can be described by Fermi's golden rule.[4] The rate of emission depends on two factors: an 'atomic part', which describes the internal structure of the light source and a 'field part', which describes the density of electromagnetic modes of the environment. The atomic part describes the strength of a transition between two states in terms of transition moments. In a homogeneous medium, such as free space, the rate of spontaneous emission in the dipole approximation is given by:
where is the emission frequency, is the index of refraction, is the transition dipole moment, is the vacuum permittivity, is the reduced Planck constant, is the vacuum speed of light, and is the fine structure constant. (This approximation breaks down in the case of inner shell electrons in high-Z atoms.) Clearly, the rate of spontaneous emission in free space increases with . In contrast with atoms, which have a discrete emission spectrum, quantum dots can be tuned continuously by changing their size. This property has been used to check the -frequency dependence of the spontaneous emission rate as described by Fermi's golden rule.[5]
Radiative and nonradiative decay: the quantum efficiency
In the rate-equation above, it is assumed that decay of the number of excited states only occurs under emission of light. In this case one speaks of full radiative decay and this means that the quantum efficiency is 100%. Besides radiative decay, which occurs under the emission of light, there is a second decay mechanism; nonradiative decay. To determine the total decay rate , radiative and nonradiative rates should be summed:
where is the total decay rate, is the radiative decay rate and the nonradiative decay rate. The quantum efficiency (QE) is defined as the fraction of emission processes in which emission of light is involved:
In nonradiative relaxation, the energy is released as phonons, more commonly known as heat. Nonradiative relaxation occurs when the energy difference between the levels is very small, and these typically occur on a much faster time scale than radiative transitions. For many materials (for instance, semiconductors), electrons move quickly from a high energy level to a meta-stable level via small nonradiative transitions and then make the final move down to the bottom level via an optical or radiative transition. This final transition is the transition over the bandgap in semiconductors. Large nonradiative transitions do not occur frequently because the crystal structure generally can not support large vibrations without destroying bonds (which generally doesn't happen for relaxation). Meta-stable states form a very important feature that is exploited in the construction of lasers. Specifically, since electrons decay slowly from them, they can be piled up in this state without too much loss and then stimulated emission can be used to boost an optical signal.
See also
- Absorption (optics)
- Stimulated emission
- Emission spectrum
- Spectral line
- Atomic spectral line
- Laser science
- Purcell effect
- Photonic crystal
- Vacuum Rabi oscillation
- Jaynes-Cummings model
References
43 year old Petroleum Engineer Harry from Deep River, usually spends time with hobbies and interests like renting movies, property developers in singapore new condominium and vehicle racing. Constantly enjoys going to destinations like Camino Real de Tierra Adentro.
External links
- Detail calculation of the Spontaneous Emission: Weisskopf-Wigner Theory
- Britney's Guide to Semiconductor Physics
- ↑ R. Loudon, The Quantum Theory of Light, 3rd ed. (Oxford University Press Inc., New York, 2001).
- ↑
20 year-old Real Estate Agent Rusty from Saint-Paul, has hobbies and interests which includes monopoly, property developers in singapore and poker. Will soon undertake a contiki trip that may include going to the Lower Valley of the Omo.
My blog: http://www.primaboinca.com/view_profile.php?userid=5889534 - ↑
20 year-old Real Estate Agent Rusty from Saint-Paul, has hobbies and interests which includes monopoly, property developers in singapore and poker. Will soon undertake a contiki trip that may include going to the Lower Valley of the Omo.
My blog: http://www.primaboinca.com/view_profile.php?userid=5889534 - ↑ B. Henderson and G. Imbusch, Optical Spectroscopy of Inorganic Solids (Clarendon Press, Oxford, UK, 1989).
- ↑ A. F. van Driel, G. Allan, C. Delerue, P. Lodahl,W. L. Vos and D. Vanmaekelbergh, Frequency-dependent spontaneous emission rate from CdSe and CdTe nanocrystals: Influence of dark states, Physical Review Letters, 95, 236804 (2005).http://cops.tnw.utwente.nl/pdf/05/PHYSICAL%20REVIEW%20LETTERS%2095%20236804%20(2005).pdf